| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232461 | 968792 | 2015 | 9 صفحه PDF | دانلود رایگان |
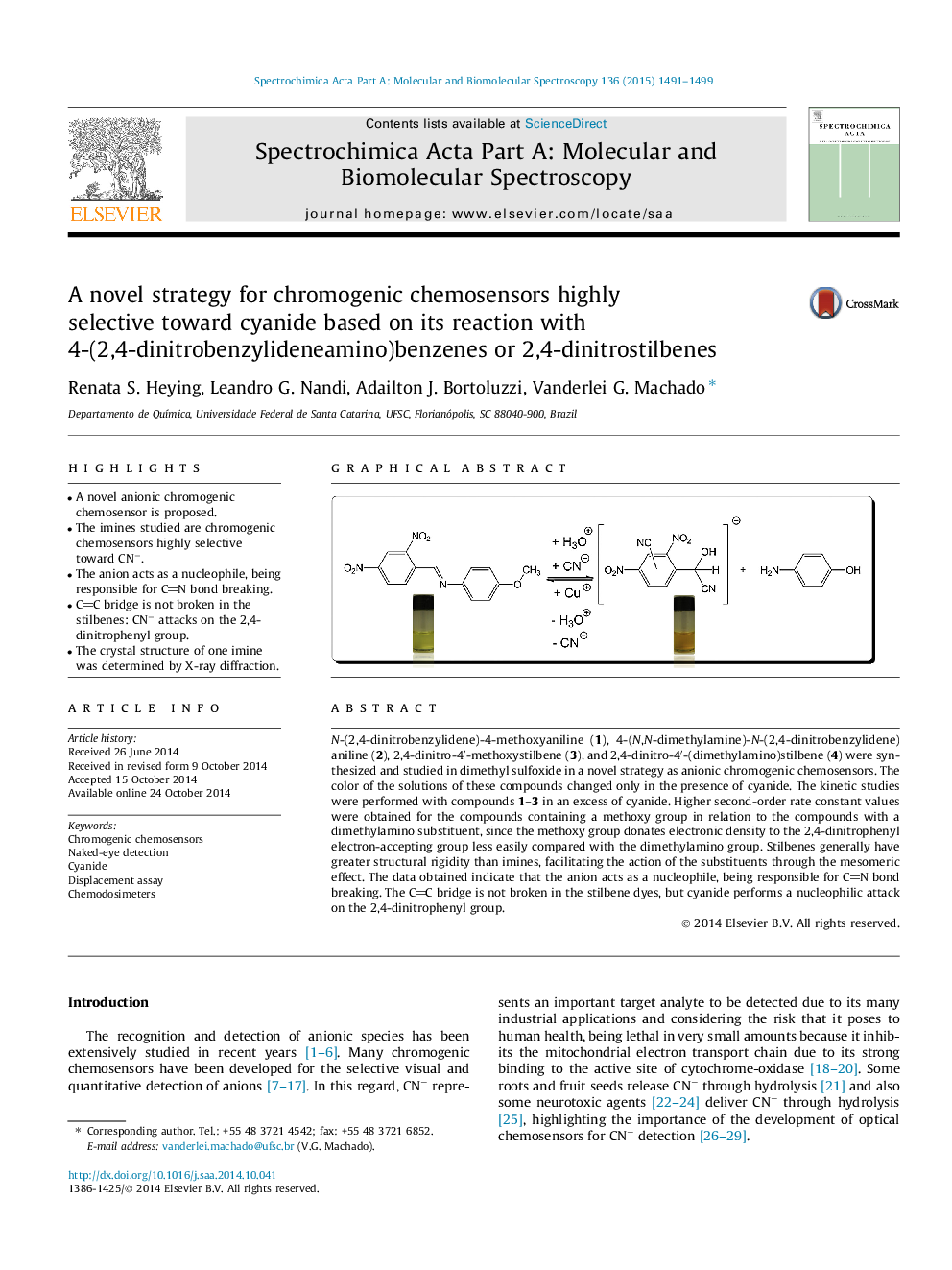
• A novel anionic chromogenic chemosensor is proposed.
• The imines studied are chromogenic chemosensors highly selective toward CN−.
• The anion acts as a nucleophile, being responsible for CN bond breaking.
• CC bridge is not broken in the stilbenes: CN− attacks on the 2,4-dinitrophenyl group.
• The crystal structure of one imine was determined by X-ray diffraction.
N-(2,4-dinitrobenzylidene)-4-methoxyaniline (1), 4-(N,N-dimethylamine)-N-(2,4-dinitrobenzylidene)aniline (2), 2,4-dinitro-4′-methoxystilbene (3), and 2,4-dinitro-4′-(dimethylamino)stilbene (4) were synthesized and studied in dimethyl sulfoxide in a novel strategy as anionic chromogenic chemosensors. The color of the solutions of these compounds changed only in the presence of cyanide. The kinetic studies were performed with compounds 1–3 in an excess of cyanide. Higher second-order rate constant values were obtained for the compounds containing a methoxy group in relation to the compounds with a dimethylamino substituent, since the methoxy group donates electronic density to the 2,4-dinitrophenyl electron-accepting group less easily compared with the dimethylamino group. Stilbenes generally have greater structural rigidity than imines, facilitating the action of the substituents through the mesomeric effect. The data obtained indicate that the anion acts as a nucleophile, being responsible for CN bond breaking. The CC bridge is not broken in the stilbene dyes, but cyanide performs a nucleophilic attack on the 2,4-dinitrophenyl group.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part C, 5 February 2015, Pages 1491–1499