| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232482 | 968792 | 2015 | 5 صفحه PDF | دانلود رایگان |
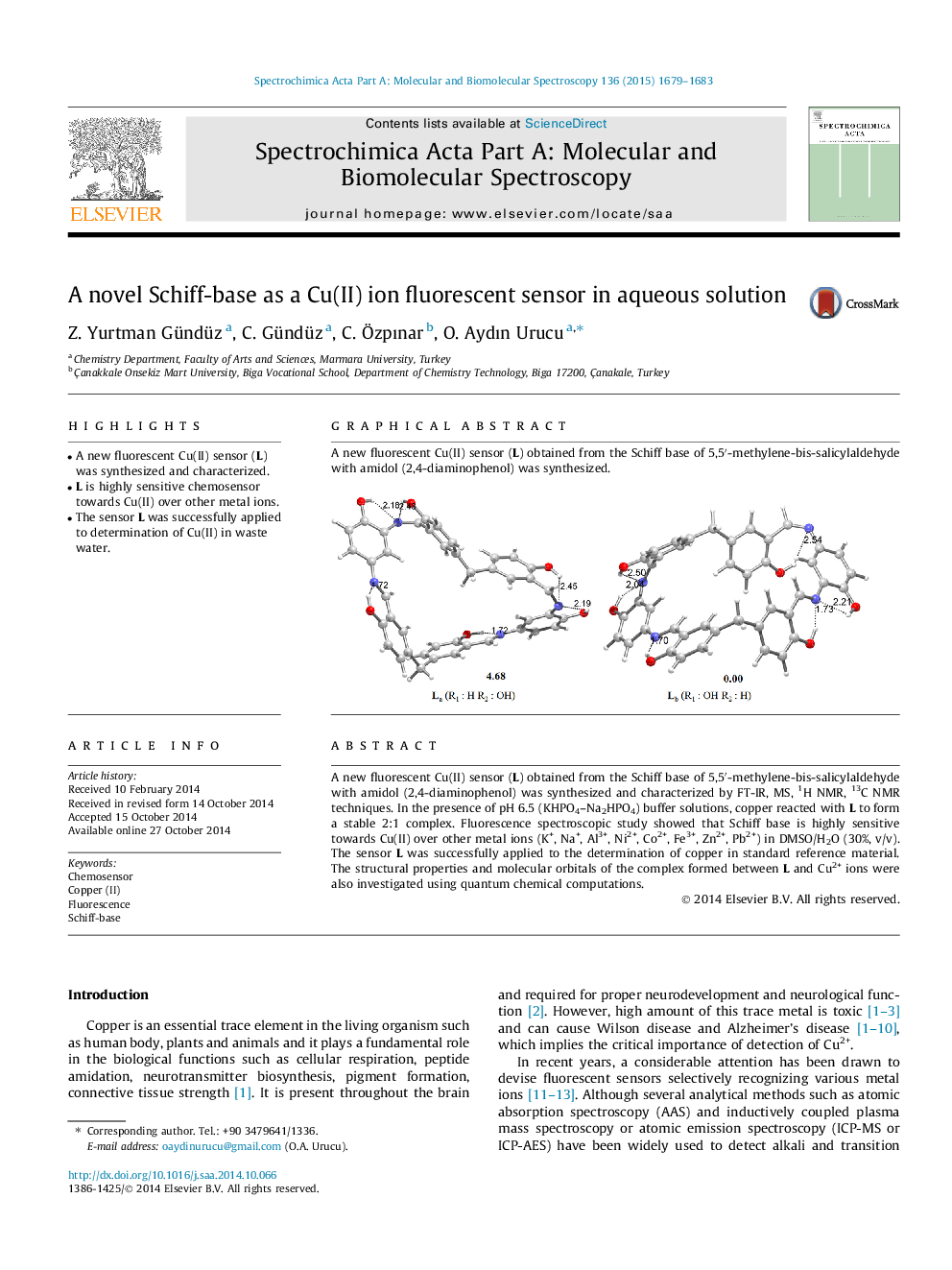
• A new fluorescent Cu(II) sensor (L) was synthesized and characterized.
• L is highly sensitive chemosensor towards Cu(II) over other metal ions.
• The sensor L was successfully applied to determination of Cu(II) in waste water.
A new fluorescent Cu(II) sensor (L) obtained from the Schiff base of 5,5′-methylene-bis-salicylaldehyde with amidol (2,4-diaminophenol) was synthesized and characterized by FT-IR, MS, 1H NMR, 13C NMR techniques. In the presence of pH 6.5 (KHPO4–Na2HPO4) buffer solutions, copper reacted with L to form a stable 2:1 complex. Fluorescence spectroscopic study showed that Schiff base is highly sensitive towards Cu(II) over other metal ions (K+, Na+, Al3+, Ni2+, Co2+, Fe3+, Zn2+, Pb2+) in DMSO/H2O (30%, v/v). The sensor L was successfully applied to the determination of copper in standard reference material. The structural properties and molecular orbitals of the complex formed between L and Cu2+ ions were also investigated using quantum chemical computations.
A new fluorescent Cu(II) sensor (L) obtained from the Schiff base of 5,5′-methylene-bis-salicylaldehyde with amidol (2,4-diaminophenol) was synthesized.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part C, 5 February 2015, Pages 1679–1683