| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232820 | 1495245 | 2014 | 12 صفحه PDF | دانلود رایگان |
• Preparation of Zn2+, Cd2+ and Hg2+ complexes of the new thiosemicarbazide.
• Elemental analysis, spectral characterization of the ligand and complexes.
• pH-metric studies in 50% dioxane–water mixture at different temperatures.
• Thermal behavior of the solid metal complexes was studied using TGA technique.
• The compounds were screened for antioxidant activity and cytotoxicity.
Hg(II), Cd(II) and binuclear Zn(II) complexes derived from the tetradentate N1-ethyl-N2-(pyridine-2-yl) hydrazine-1, 2-bis (carbothioamide) ligand (H2PET) have been prepared and characterized by conventional techniques. The isolated complexes acquired the formulas, [Hg(HPET)(H2O)2Cl]⋅H2O, [Cd(HPET)Cl] and [Zn2(HPET)(PET)(OAc)]⋅H2O, respectively. IR data revealed that the ligand behaves as monobasic tridentate through (CN)py, (C–S) and new (NC)azomethine∗ groups in both Hg(II) and Cd(II) complexes. In the binuclear Zn(II) complex, the behavior of ligand contains two types, where H2PET acts as dibasic tetradentate via (CN)py, both deprotonated (C–SH) and the new (NC)azomethine∗ towards two Zn atoms and also it acts as monobasic tridentate via (CS), deprotonated (C–SH) and (CN)py towards the same Zn atoms. An octahedral geometry for Hg(II) complex and tetrahedral geometry for both Cd(II) and Zn(II) complexes were proposed. The bond lengths, bond angles, HOMO, LUMO and dipole moment have been calculated by DFT using materials studio program to confirm the geometry of ligand and its metal complexes. The association constant of the ligand and the stability constants of its complexes as well as the thermodynamic parameters were calculated by pH metric measurements at 298, 308 and 318 K in 50% dioxane–water mixture, respectively. Also, the kinetic and thermodynamic parameters for the different thermal degradation steps of the complexes were determined by Coats-Redfern and Horowitz–Metzger methods. Moreover, the anti-oxidant (using ABTS and DPPH methods), anti-hemolytic, and cytotoxic activities of the compounds have been tested.
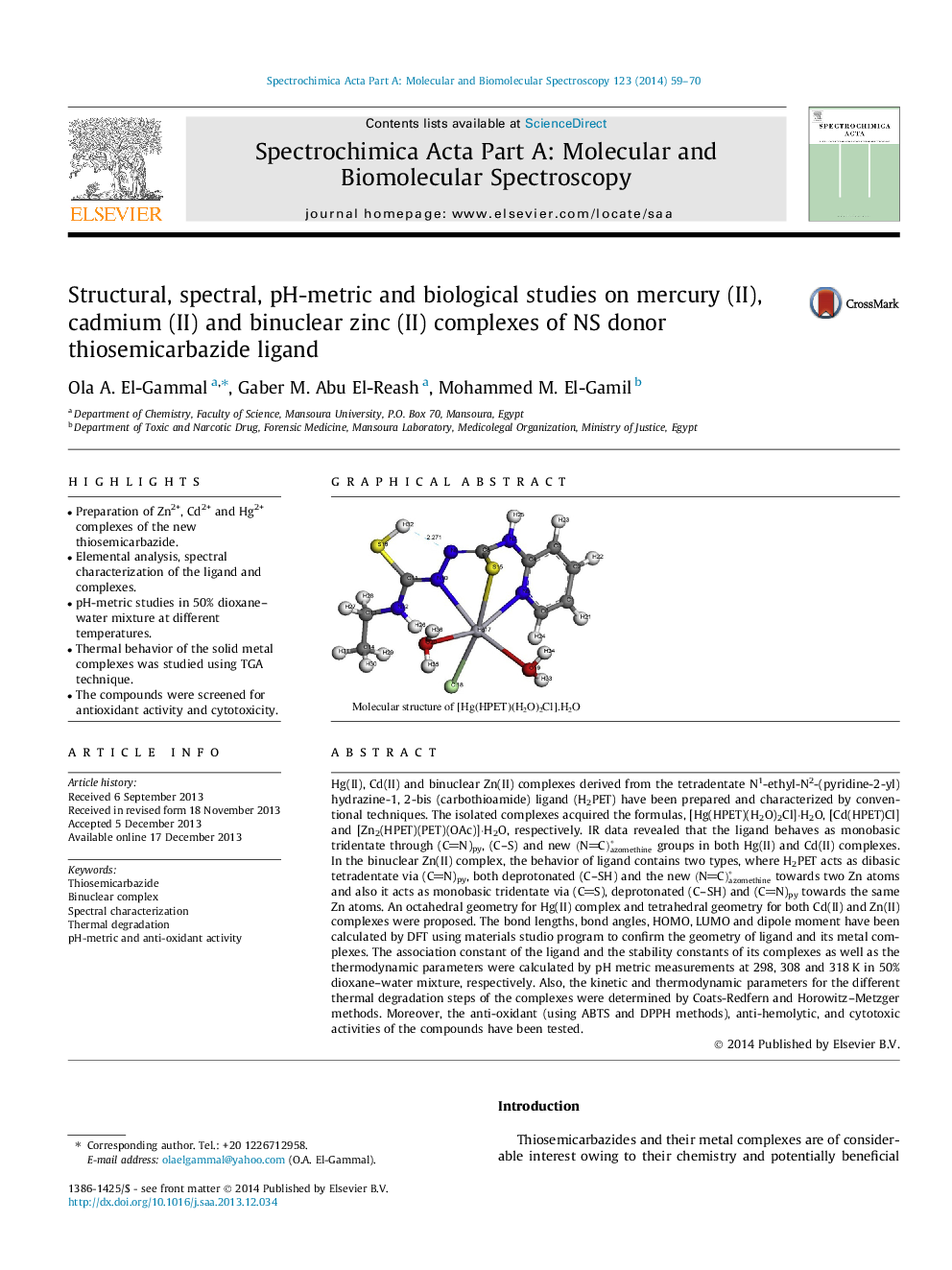
Molecular structure of [Hg(HPET)(H2O)2Cl]⋅H2O.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 123, 5 April 2014, Pages 59–70