| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232848 | 1495245 | 2014 | 8 صفحه PDF | دانلود رایگان |
• The conformational behavior was studied by ab initio and DFT calculations.
• FTIR and Raman investigation of 2,2,2-trichloroethylacetate were carried out.
• The fundamental vibrational modes were assigned.
• NBO and AIM analysis were performed in order to investigate the conformational preference.
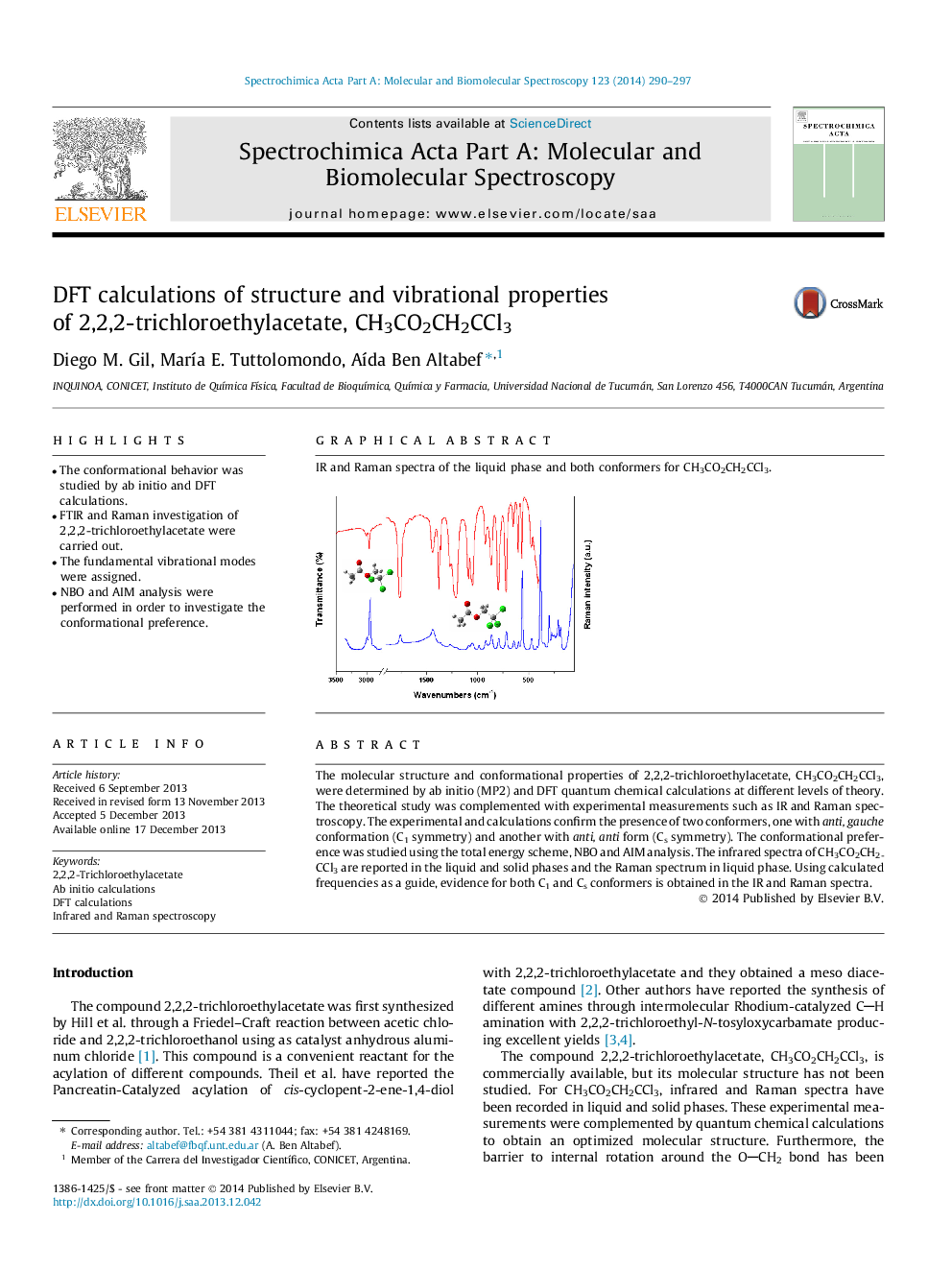
The molecular structure and conformational properties of 2,2,2-trichloroethylacetate, CH3CO2CH2CCl3, were determined by ab initio (MP2) and DFT quantum chemical calculations at different levels of theory. The theoretical study was complemented with experimental measurements such as IR and Raman spectroscopy. The experimental and calculations confirm the presence of two conformers, one with anti, gauche conformation (C1 symmetry) and another with anti, anti form (Cs symmetry). The conformational preference was studied using the total energy scheme, NBO and AIM analysis. The infrared spectra of CH3CO2CH2CCl3 are reported in the liquid and solid phases and the Raman spectrum in liquid phase. Using calculated frequencies as a guide, evidence for both C1 and Cs conformers is obtained in the IR and Raman spectra.
IR and Raman spectra of the liquid phase and both conformers for CH3CO2CH2CCl3.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 123, 5 April 2014, Pages 290–297