| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232953 | 968800 | 2015 | 5 صفحه PDF | دانلود رایگان |
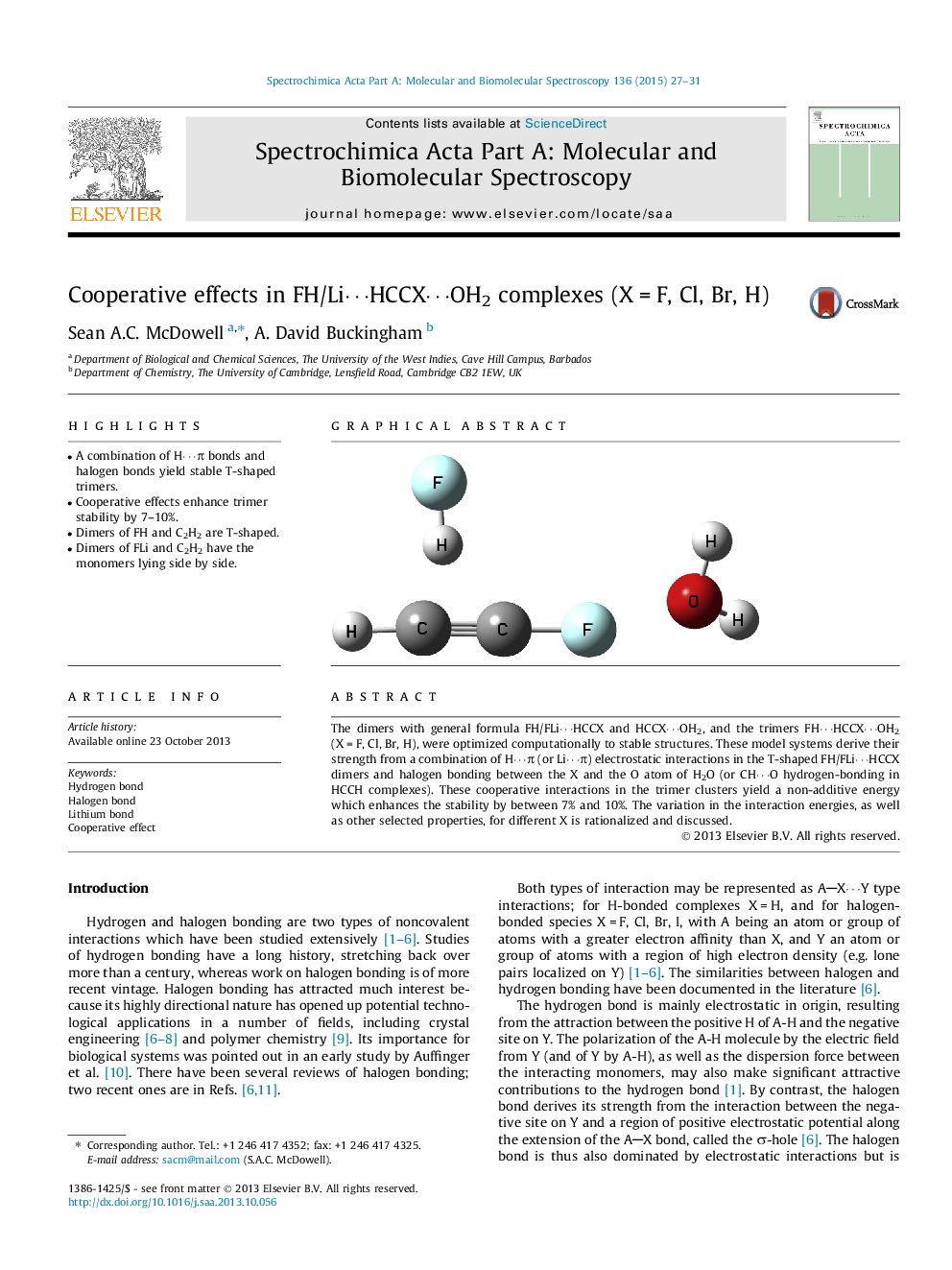
• A combination of H⋯π bonds and halogen bonds yield stable T-shaped trimers.
• Cooperative effects enhance trimer stability by 7–10%.
• Dimers of FH and C2H2 are T-shaped.
• Dimers of FLi and C2H2 have the monomers lying side by side.
The dimers with general formula FH/FLi⋯HCCX and HCCX⋯OH2, and the trimers FH⋯HCCX⋯OH2 (X = F, Cl, Br, H), were optimized computationally to stable structures. These model systems derive their strength from a combination of H⋯π (or Li⋯π) electrostatic interactions in the T-shaped FH/FLi⋯HCCX dimers and halogen bonding between the X and the O atom of H2O (or CH⋯O hydrogen-bonding in HCCH complexes). These cooperative interactions in the trimer clusters yield a non-additive energy which enhances the stability by between 7% and 10%. The variation in the interaction energies, as well as other selected properties, for different X is rationalized and discussed.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part A, 5 February 2015, Pages 27–31