| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233205 | 1495233 | 2015 | 5 صفحه PDF | دانلود رایگان |
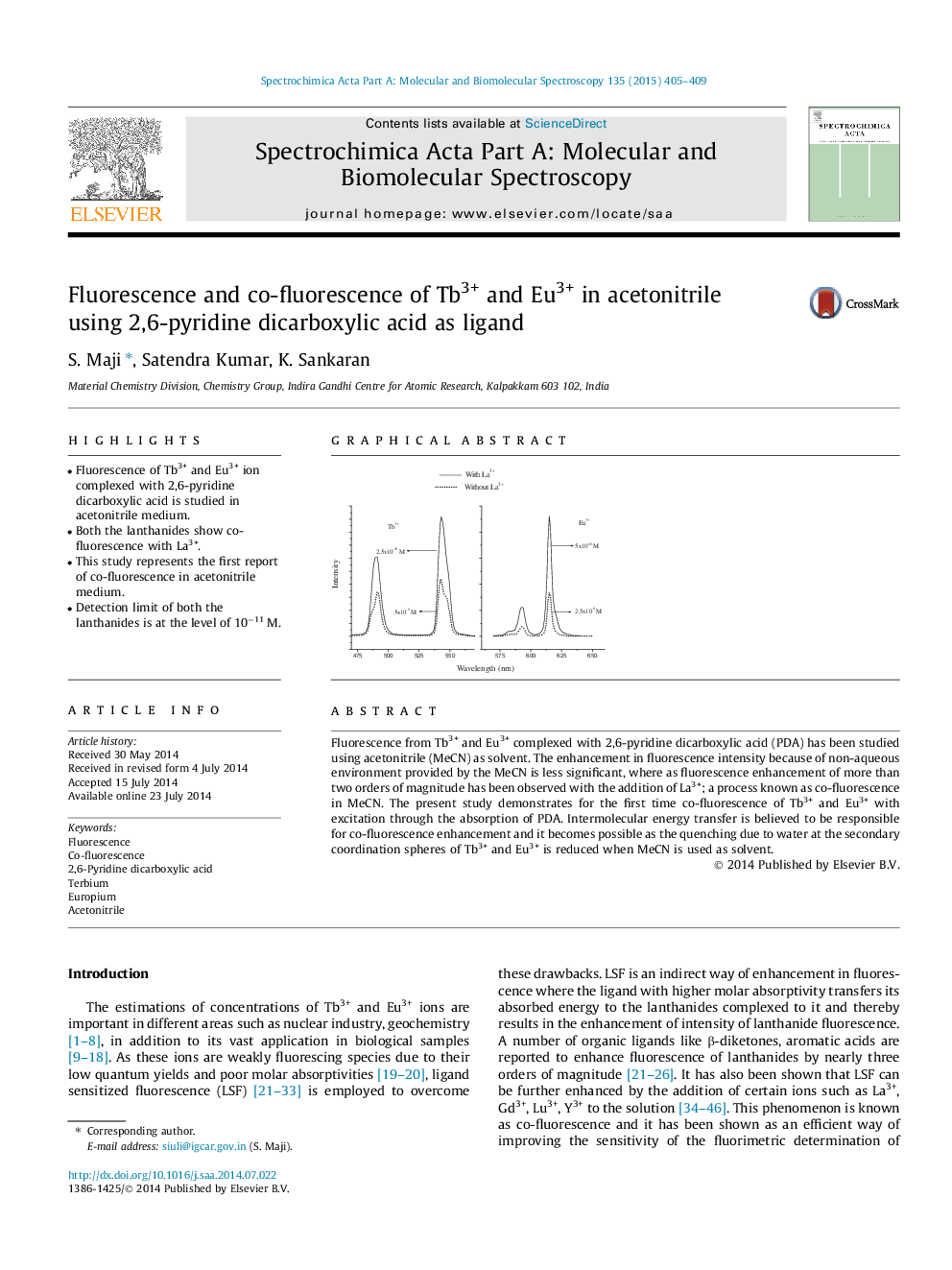
• Fluorescence of Tb3+ and Eu3+ ion complexed with 2,6-pyridine dicarboxylic acid is studied in acetonitrile medium.
• Both the lanthanides show co-fluorescence with La3+.
• This study represents the first report of co-fluorescence in acetonitrile medium.
• Detection limit of both the lanthanides is at the level of 10−11 M.
Fluorescence from Tb3+ and Eu3+ complexed with 2,6-pyridine dicarboxylic acid (PDA) has been studied using acetonitrile (MeCN) as solvent. The enhancement in fluorescence intensity because of non-aqueous environment provided by the MeCN is less significant, where as fluorescence enhancement of more than two orders of magnitude has been observed with the addition of La3+; a process known as co-fluorescence in MeCN. The present study demonstrates for the first time co-fluorescence of Tb3+ and Eu3+ with excitation through the absorption of PDA. Intermolecular energy transfer is believed to be responsible for co-fluorescence enhancement and it becomes possible as the quenching due to water at the secondary coordination spheres of Tb3+ and Eu3+ is reduced when MeCN is used as solvent.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 135, 25 January 2015, Pages 405–409