| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233469 | 1495235 | 2014 | 7 صفحه PDF | دانلود رایگان |
• Iron(III)–oxazoline complex is applied in oxidation of sulfides.
• Urea hydrogen peroxide (UHP) as oxidant.
• Excellent yield and selectivity has been achieved.
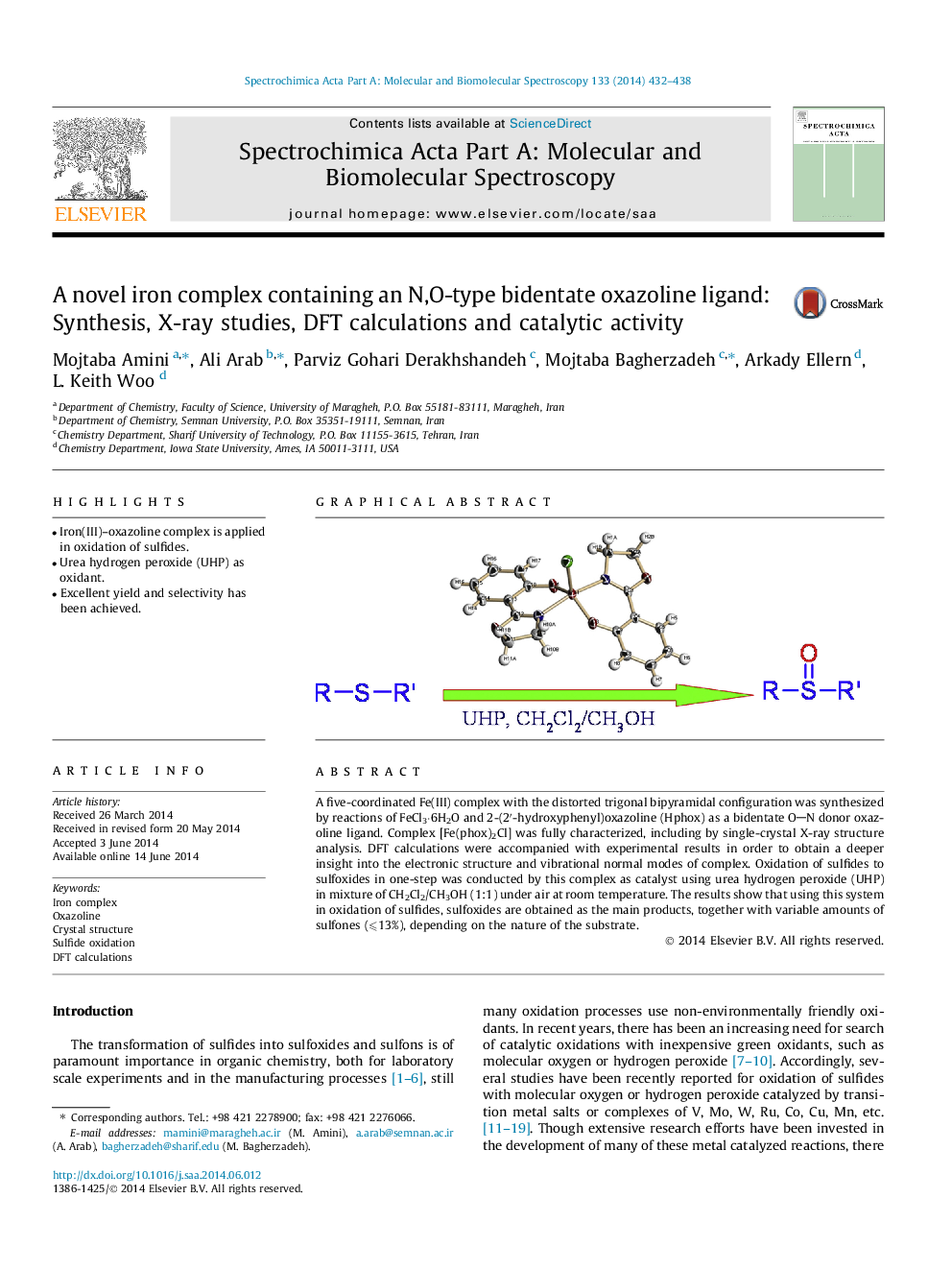
A five-coordinated Fe(III) complex with the distorted trigonal bipyramidal configuration was synthesized by reactions of FeCl3⋅6H2O and 2-(2′-hydroxyphenyl)oxazoline (Hphox) as a bidentate ON donor oxazoline ligand. Complex [Fe(phox)2Cl] was fully characterized, including by single-crystal X-ray structure analysis. DFT calculations were accompanied with experimental results in order to obtain a deeper insight into the electronic structure and vibrational normal modes of complex. Oxidation of sulfides to sulfoxides in one-step was conducted by this complex as catalyst using urea hydrogen peroxide (UHP) in mixture of CH2Cl2/CH3OH (1:1) under air at room temperature. The results show that using this system in oxidation of sulfides, sulfoxides are obtained as the main products, together with variable amounts of sulfones (⩽13%), depending on the nature of the substrate.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 133, 10 December 2014, Pages 432–438