| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233952 | 1495241 | 2014 | 7 صفحه PDF | دانلود رایگان |
• Monomeric Mn (IV) and pseudo-tetrameric Mn (III) complexes have been synthesized.
• Crystal structures have been determined by single crystal X-ray diffraction analysis.
• Magnetic susceptibilities have been measured over the temperature range 2–300 K for Mn (III) complex.
• Magnetic parameters have been determined with fitting procedure.
• Magnetic results indicate antiferromagnetic interaction between metal ions for Mn (III) complex.
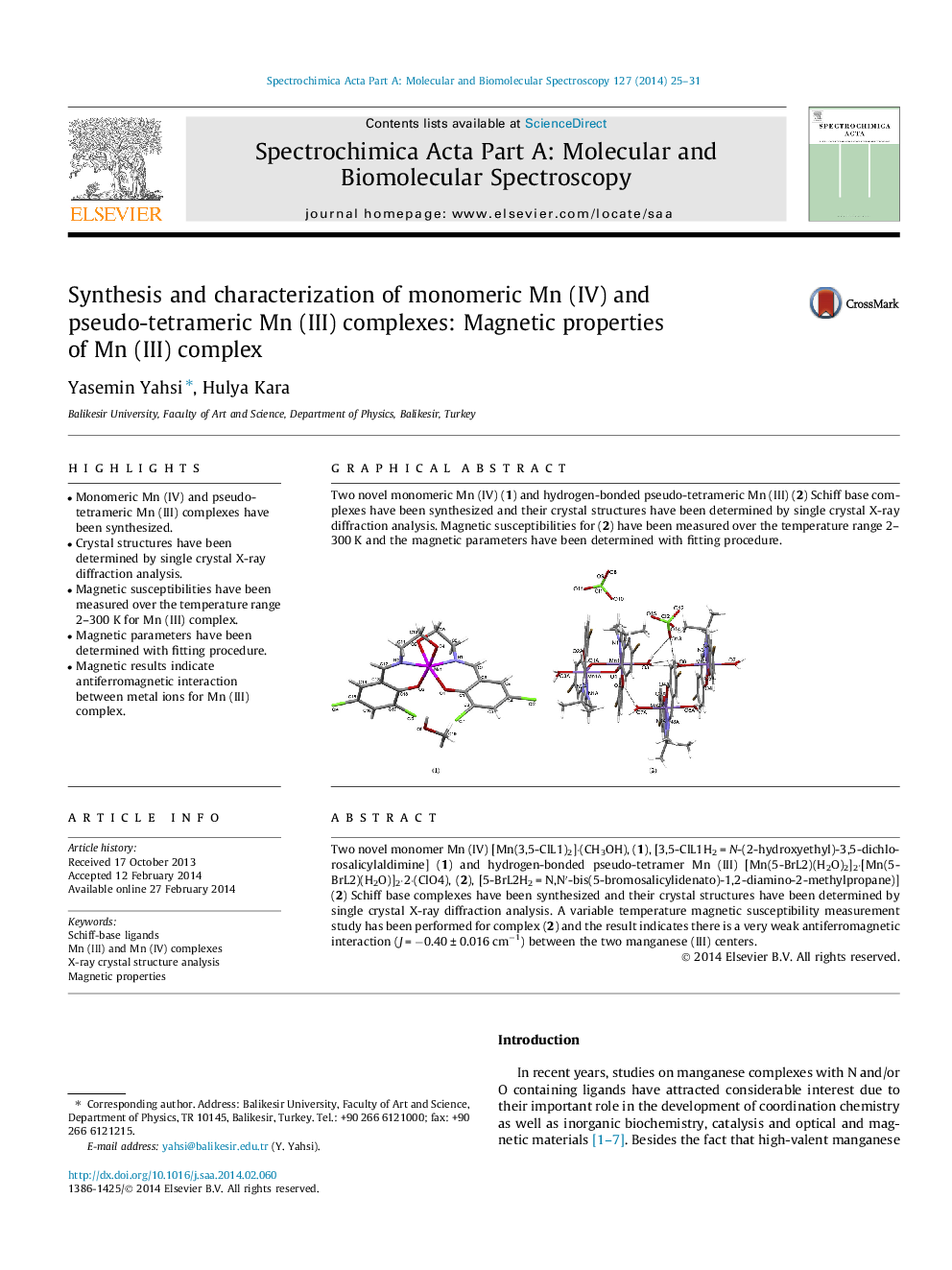
Two novel monomer Mn (IV) [Mn(3,5-ClL1)2]⋅(CH3OH), (1), [3,5-ClL1H2 = N-(2-hydroxyethyl)-3,5-dichlorosalicylaldimine] (1) and hydrogen-bonded pseudo-tetramer Mn (III) [Mn(5-BrL2)(H2O)2]2⋅[Mn(5-BrL2)(H2O)]2⋅2⋅(ClO4), (2), [5-BrL2H2 = N,N′-bis(5-bromosalicylidenato)-1,2-diamino-2-methylpropane)] (2) Schiff base complexes have been synthesized and their crystal structures have been determined by single crystal X-ray diffraction analysis. A variable temperature magnetic susceptibility measurement study has been performed for complex (2) and the result indicates there is a very weak antiferromagnetic interaction (J = −0.40 ± 0.016 cm−1) between the two manganese (III) centers.
Two novel monomeric Mn (IV) (1) and hydrogen-bonded pseudo-tetrameric Mn (III) (2) Schiff base complexes have been synthesized and their crystal structures have been determined by single crystal X-ray diffraction analysis. Magnetic susceptibilities for (2) have been measured over the temperature range 2–300 K and the magnetic parameters have been determined with fitting procedure.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 127, 5 June 2014, Pages 25–31