| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1234429 | 1495272 | 2012 | 12 صفحه PDF | دانلود رایگان |
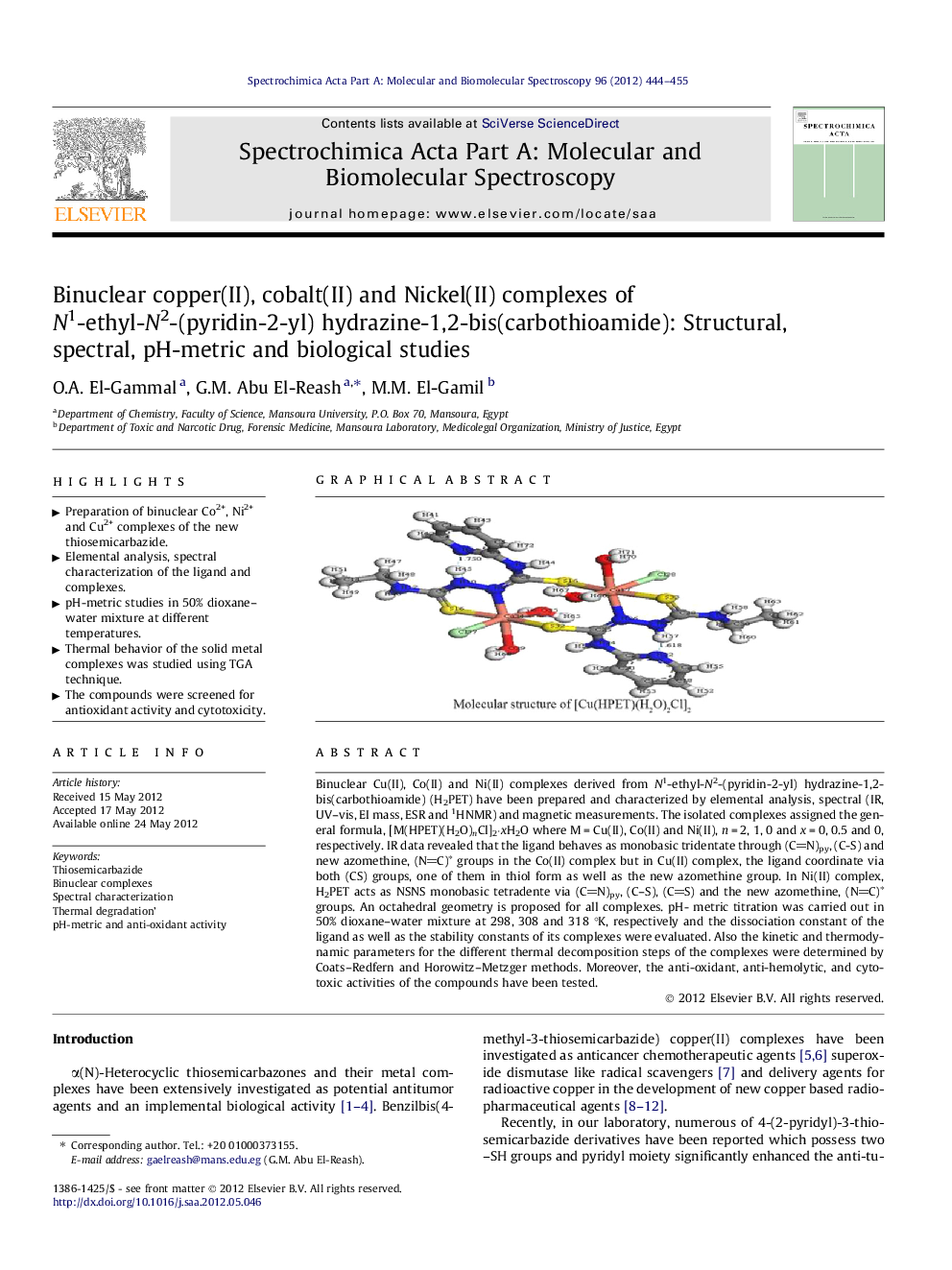
Binuclear Cu(II), Co(II) and Ni(II) complexes derived from N1-ethyl-N2-(pyridin-2-yl) hydrazine-1,2-bis(carbothioamide) (H2PET) have been prepared and characterized by elemental analysis, spectral (IR, UV–vis, EI mass, ESR and 1HNMR) and magnetic measurements. The isolated complexes assigned the general formula, [M(HPET)(H2O)nCl]2·xH2O where M = Cu(II), Co(II) and Ni(II), n = 2, 1, 0 and x = 0, 0.5 and 0, respectively. IR data revealed that the ligand behaves as monobasic tridentate through (CN)py, (C-S) and new azomethine, (NC)∗ groups in the Co(II) complex but in Cu(II) complex, the ligand coordinate via both (CS) groups, one of them in thiol form as well as the new azomethine group. In Ni(II) complex, H2PET acts as NSNS monobasic tetradente via (CN)py, (C–S), (CS) and the new azomethine, (NC)∗ groups. An octahedral geometry is proposed for all complexes. pH- metric titration was carried out in 50% dioxane–water mixture at 298, 308 and 318 °K, respectively and the dissociation constant of the ligand as well as the stability constants of its complexes were evaluated. Also the kinetic and thermodynamic parameters for the different thermal decomposition steps of the complexes were determined by Coats–Redfern and Horowitz–Metzger methods. Moreover, the anti-oxidant, anti-hemolytic, and cytotoxic activities of the compounds have been tested.
Figure optionsDownload as PowerPoint slideHighlights
► Preparation of binuclear Co2+, Ni2+ and Cu2+ complexes of the new thiosemicarbazide.
► Elemental analysis, spectral characterization of the ligand and complexes.
► pH-metric studies in 50% dioxane–water mixture at different temperatures.
► Thermal behavior of the solid metal complexes was studied using TGA technique.
► The compounds were screened for antioxidant activity and cytotoxicity.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 96, October 2012, Pages 444–455