| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1234768 | 1495260 | 2013 | 5 صفحه PDF | دانلود رایگان |
Charge transfer complexes of electron deficient (dibenzoylmethanato)boron difluoride (DBMBF2) with polyaromatic hydrocarbons (A), as well as with highly electron rich indeno-pyridines (I) compounds in ethanol medium have been studied by electronic absorption spectroscopy. Absorption band due to a charge-transfer (CT) transitions are observed in the visible region. Utilizing the CT transition energy, the vertical electron affinities (EAv) of DBMBF2 in ethanol has been calculated. The value of EAv for DBMBF2 is found to be 2.28 eV, this is the first report of its electron affinity value. We have calculated the degrees of CT and transition dipole strengths of the DBMBF2/A and DBMBF2/I complexes. Along with both theoretically calculated and experimentally obtained vertical ionization potentials of the indeno-pyridine donors have been estimated for the first time. I show high degree of charge transfer along with high ground state stability similar to that of A. Thus indeno-pyridine (I) donors are also as good as aromatic hydrocarbons (A) for DBMBF2, having equivalent ionization potential like that of aromatic hydrocarbons.
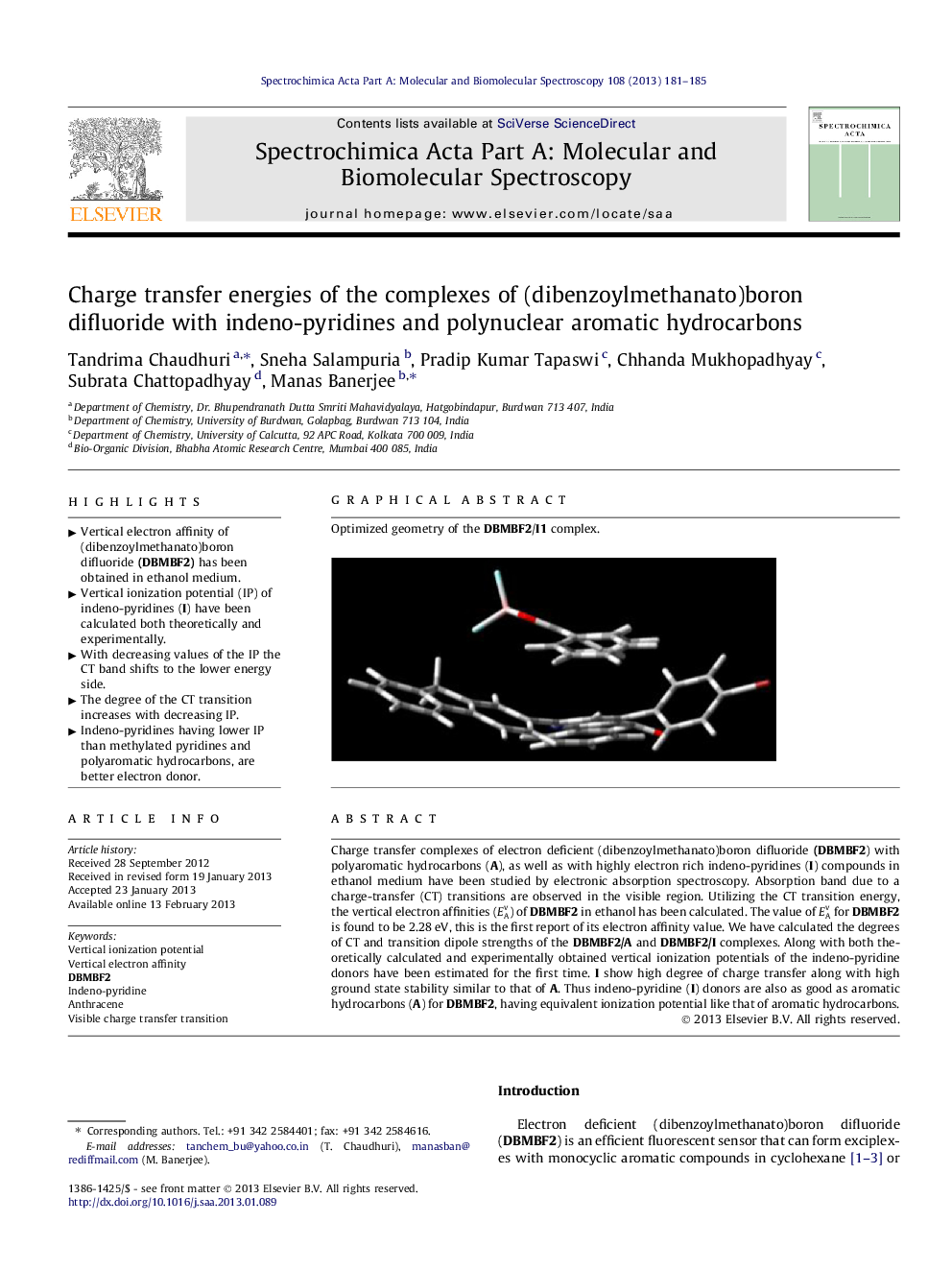
Optimized geometry of the DBMBF2/I1 complex.Figure optionsDownload as PowerPoint slideHighlights
► Vertical electron affinity of (dibenzoylmethanato)boron difluoride (DBMBF2) has been obtained in ethanol medium.
► Vertical ionization potential (IP) of indeno-pyridines (I) have been calculated both theoretically and experimentally.
► With decreasing values of the IP the CT band shifts to the lower energy side. The degree of the CT transition increases with decreasing IP.
► Indeno-pyridines having lower IP than methylated pyridines and polyaromatic hydrocarbons, are better electron donor.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 108, May 2013, Pages 181–185