| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1286078 | 1497942 | 2015 | 9 صفحه PDF | دانلود رایگان |
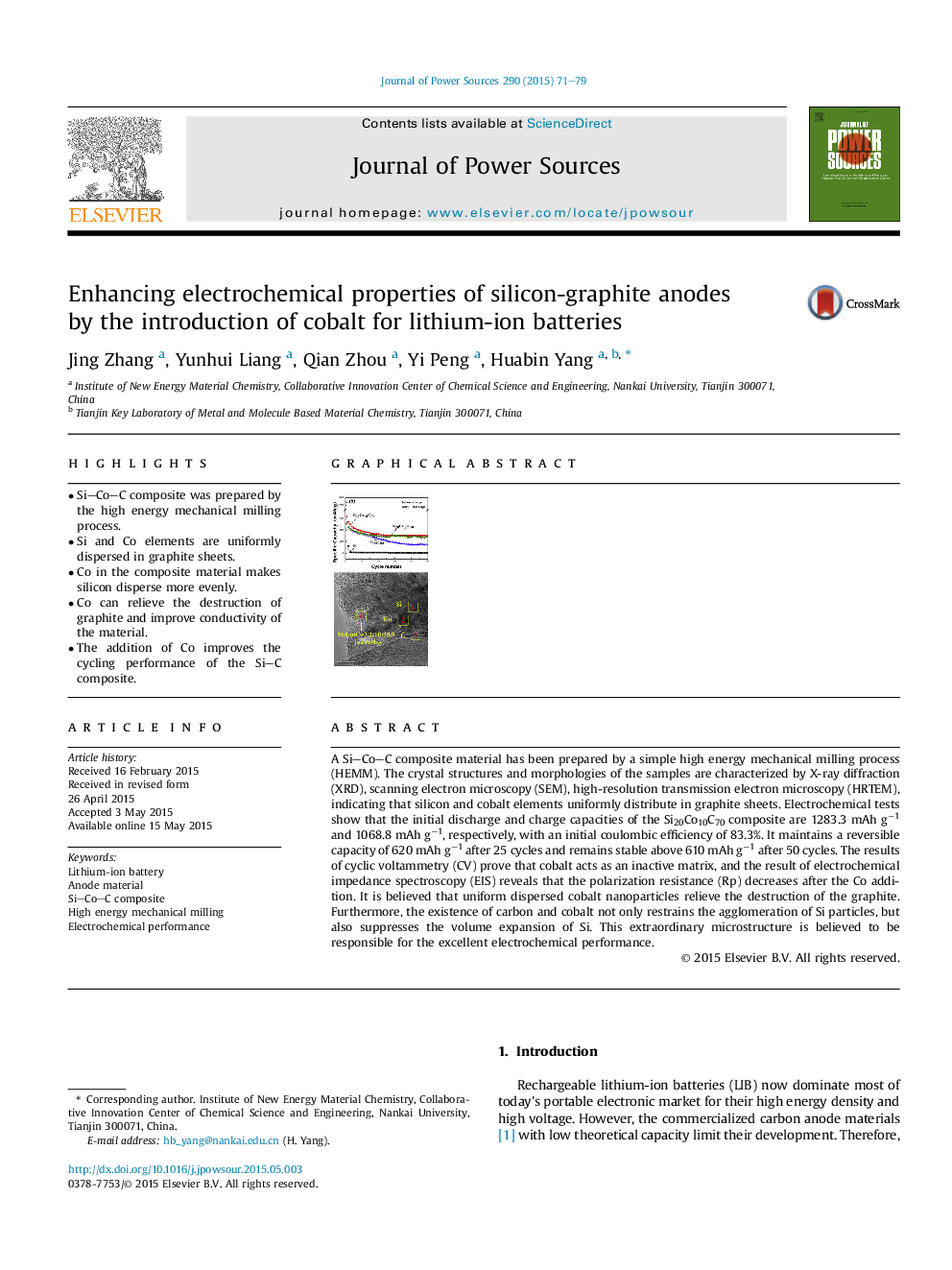
• Si–Co–C composite was prepared by the high energy mechanical milling process.
• Si and Co elements are uniformly dispersed in graphite sheets.
• Co in the composite material makes silicon disperse more evenly.
• Co can relieve the destruction of graphite and improve conductivity of the material.
• The addition of Co improves the cycling performance of the Si–C composite.
A Si–Co–C composite material has been prepared by a simple high energy mechanical milling process (HEMM). The crystal structures and morphologies of the samples are characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), indicating that silicon and cobalt elements uniformly distribute in graphite sheets. Electrochemical tests show that the initial discharge and charge capacities of the Si20Co10C70 composite are 1283.3 mAh g−1 and 1068.8 mAh g−1, respectively, with an initial coulombic efficiency of 83.3%. It maintains a reversible capacity of 620 mAh g−1 after 25 cycles and remains stable above 610 mAh g−1 after 50 cycles. The results of cyclic voltammetry (CV) prove that cobalt acts as an inactive matrix, and the result of electrochemical impedance spectroscopy (EIS) reveals that the polarization resistance (Rp) decreases after the Co addition. It is believed that uniform dispersed cobalt nanoparticles relieve the destruction of the graphite. Furthermore, the existence of carbon and cobalt not only restrains the agglomeration of Si particles, but also suppresses the volume expansion of Si. This extraordinary microstructure is believed to be responsible for the excellent electrochemical performance.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 290, 20 September 2015, Pages 71–79