| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1287963 | 1497998 | 2013 | 7 صفحه PDF | دانلود رایگان |
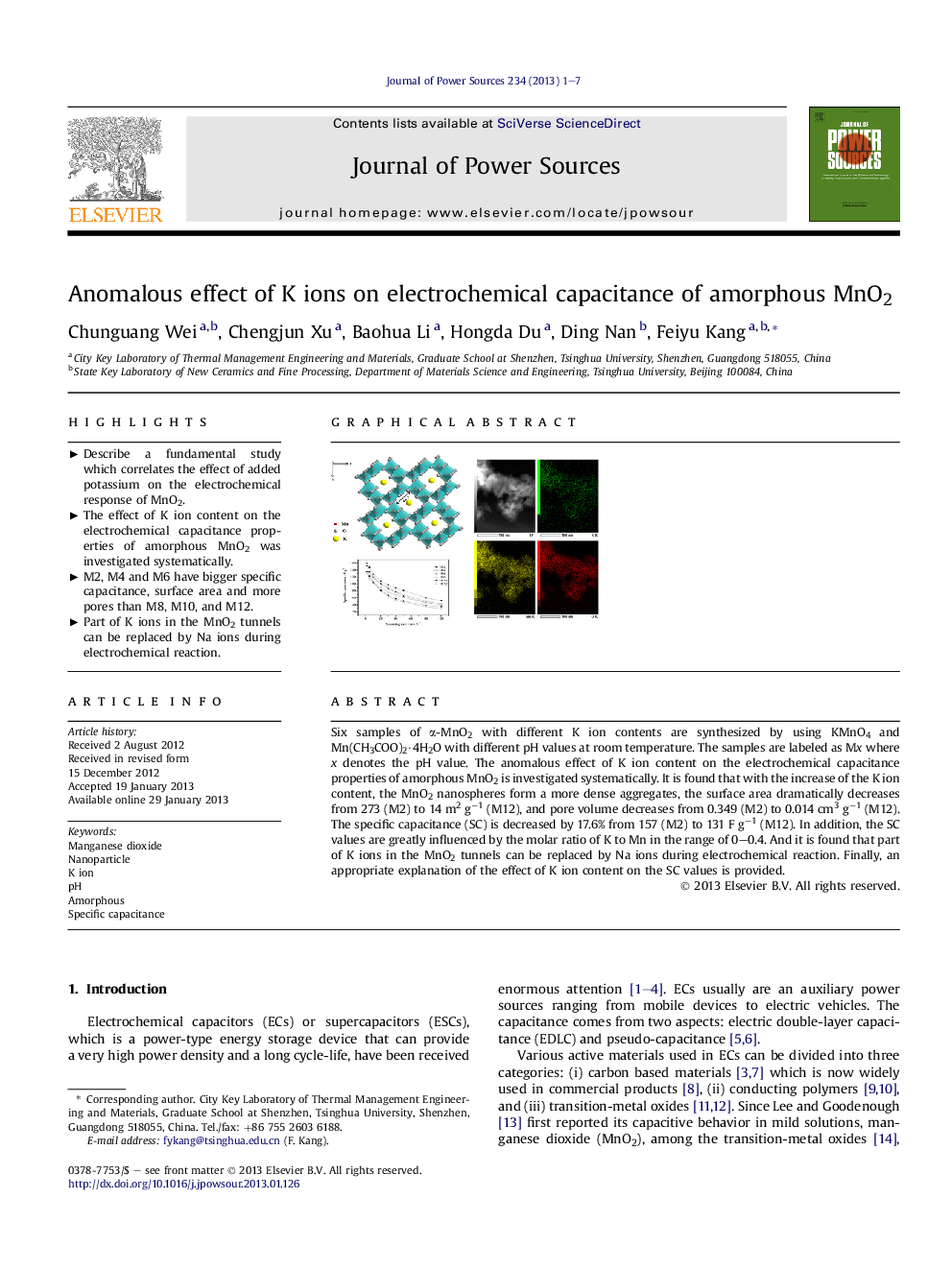
Six samples of α-MnO2 with different K ion contents are synthesized by using KMnO4 and Mn(CH3COO)2·4H2O with different pH values at room temperature. The samples are labeled as Mx where x denotes the pH value. The anomalous effect of K ion content on the electrochemical capacitance properties of amorphous MnO2 is investigated systematically. It is found that with the increase of the K ion content, the MnO2 nanospheres form a more dense aggregates, the surface area dramatically decreases from 273 (M2) to 14 m2 g−1 (M12), and pore volume decreases from 0.349 (M2) to 0.014 cm3 g−1 (M12). The specific capacitance (SC) is decreased by 17.6% from 157 (M2) to 131 F g−1 (M12). In addition, the SC values are greatly influenced by the molar ratio of K to Mn in the range of 0–0.4. And it is found that part of K ions in the MnO2 tunnels can be replaced by Na ions during electrochemical reaction. Finally, an appropriate explanation of the effect of K ion content on the SC values is provided.
Figure optionsDownload as PowerPoint slideHighlights
► Describe a fundamental study which correlates the effect of added potassium on the electrochemical response of MnO2.
► The effect of K ion content on the electrochemical capacitance properties of amorphous MnO2 was investigated systematically.
► M2, M4 and M6 have bigger specific capacitance, surface area and more pores than M8, M10, and M12.
► Part of K ions in the MnO2 tunnels can be replaced by Na ions during electrochemical reaction.
Journal: Journal of Power Sources - Volume 234, 15 July 2013, Pages 1–7