| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1334552 | 1500281 | 2013 | 5 صفحه PDF | دانلود رایگان |
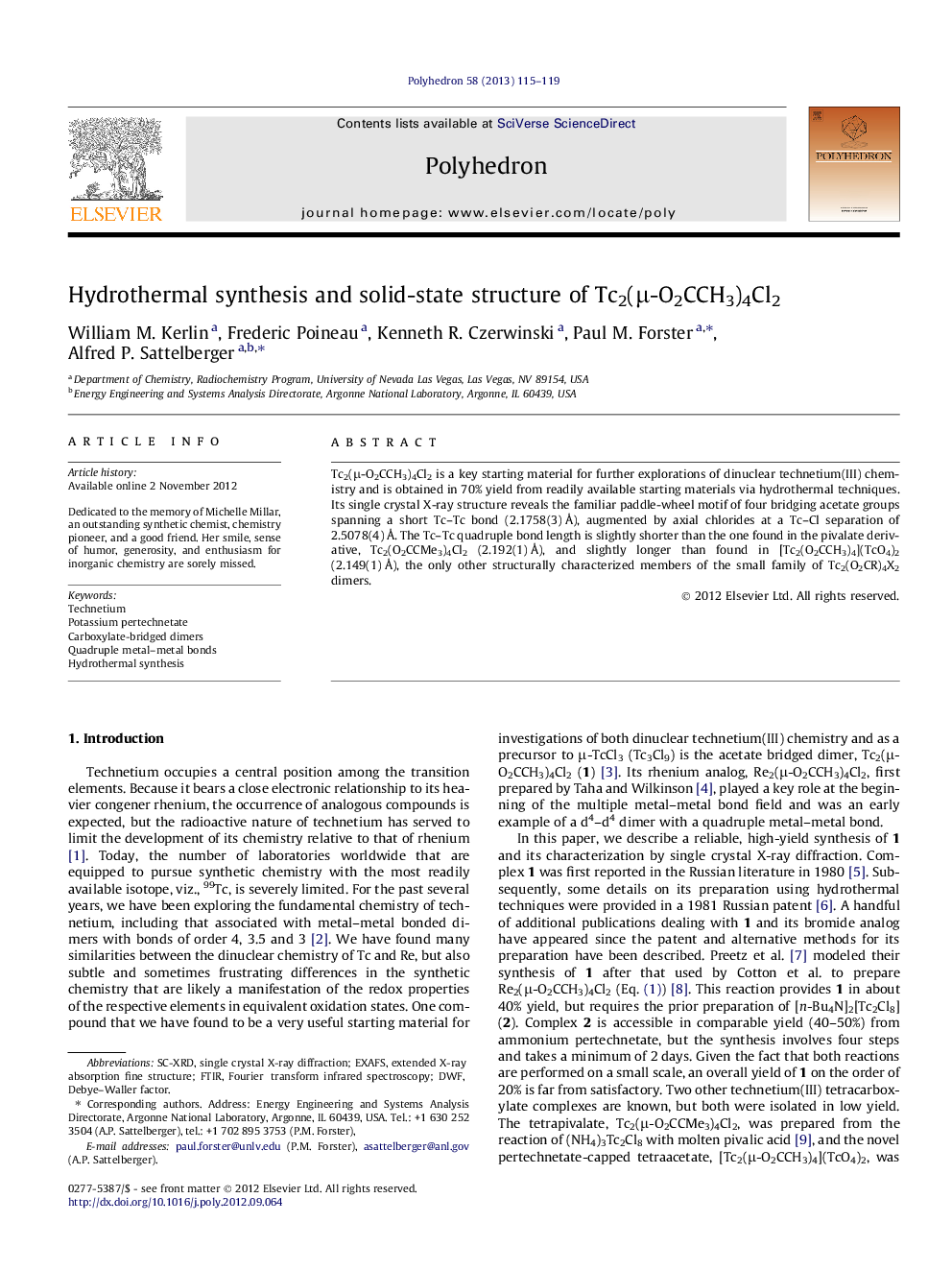
Tc2(μ-O2CCH3)4Cl2 is a key starting material for further explorations of dinuclear technetium(III) chemistry and is obtained in 70% yield from readily available starting materials via hydrothermal techniques. Its single crystal X-ray structure reveals the familiar paddle-wheel motif of four bridging acetate groups spanning a short Tc–Tc bond (2.1758(3) Å), augmented by axial chlorides at a Tc–Cl separation of 2.5078(4) Å. The Tc–Tc quadruple bond length is slightly shorter than the one found in the pivalate derivative, Tc2(O2CCMe3)4Cl2 (2.192(1) Å), and slightly longer than found in [Tc2(O2CCH3)4](TcO4)2 (2.149(1) Å), the only other structurally characterized members of the small family of Tc2(O2CR)4X2 dimers.
The quadruply metal–metal bonded technetium(III) dimer, Tc2(μ-O2CCH3)4Cl2 (1), was isolated in high yield from the reduction of potassium pertechnetate with molecular hydrogen in a mixture of acetic and hydrochloric acids under hydrothermal conditions. Red crystals of 1 covered in Paratone oil are pictured on the left and a drawing of the structure is shown on the right with key distances.Figure optionsDownload as PowerPoint slide
Journal: Polyhedron - Volume 58, 13 July 2013, Pages 115–119