| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1371263 | 981841 | 2012 | 4 صفحه PDF | دانلود رایگان |
A series of acrylamide analogues were designed and synthesized from Imatinib and Nilotinib as novel BCR–ABL inhibitors by application of the principle of nonclassical electronic isostere. All new compounds were evaluated for their inhibitory effects on the activity of BCR–ABL kinase and the proliferation of K562 leukemia cancer cells in vitro. The acrylamide analogues in which the substituent in C ring was trifluoromethyl group were identified as highly potent BCR–ABL kinase inhibitors. Compound 13f exhibited an IC50 value as low as 20.6 nM in ABL kinase inhibition and an IC50 value of 32.3 nM for antiproliferative activity, about 10.5-fold and 12-fold lower than those of Imatinib respectively. These results suggest that compound 13f is a promising candidate as a novel BCR–ABL kinase inhibitor for further development.
Novel acrylamide derivatives were synthesized and evaluated using ABL kinase and K562 cells. Introduction of trifluoromethyl group at R1 or R2 was able to enhance Imatinib efficacy in particular, the IC50 values of compound 13f were >10 times lower than those of Imatinib in both enzyme and cellular assays.Figure optionsDownload as PowerPoint slide
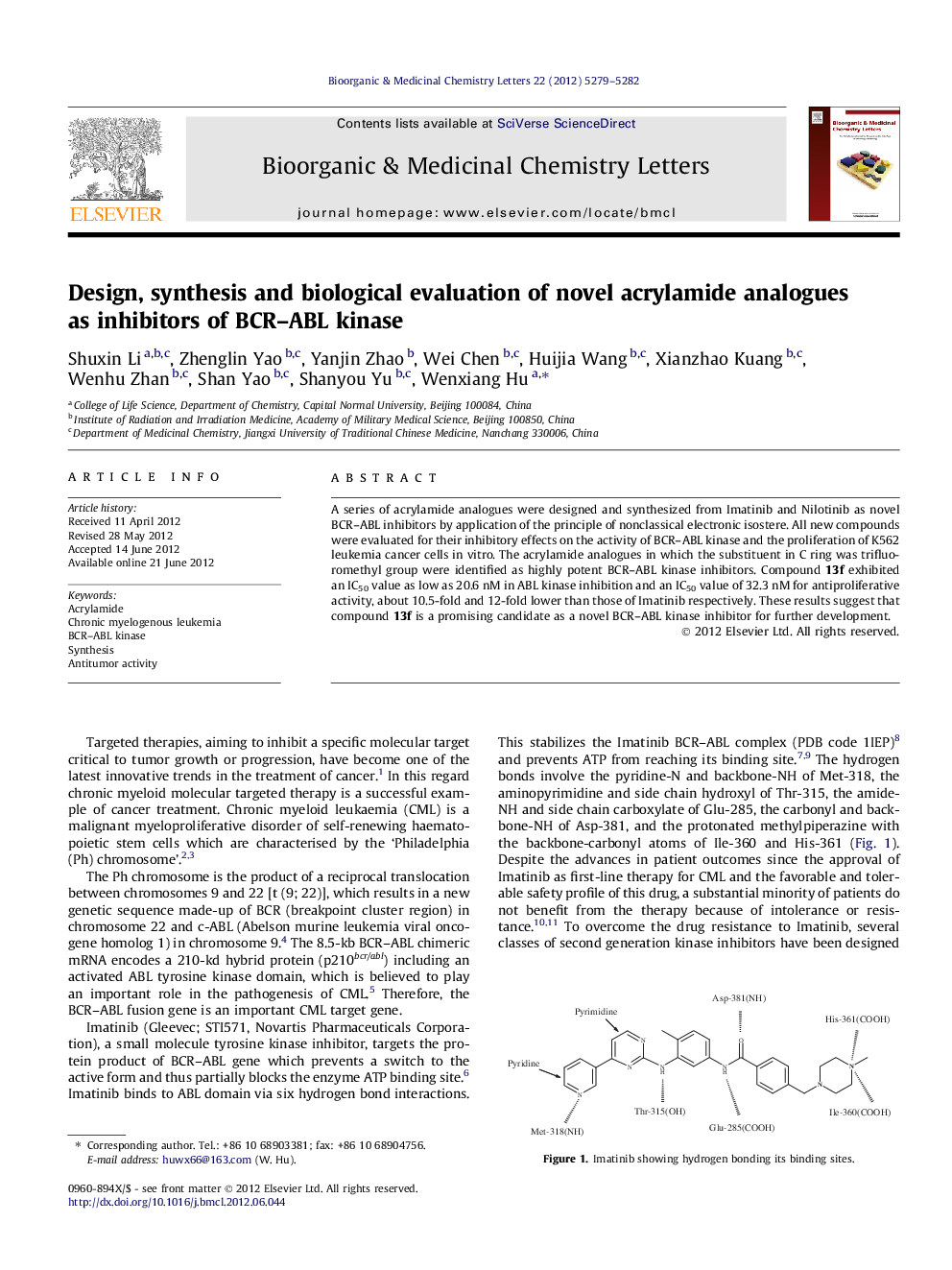
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 22, Issue 16, 15 August 2012, Pages 5279–5282