| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1371599 | 981849 | 2010 | 5 صفحه PDF | دانلود رایگان |
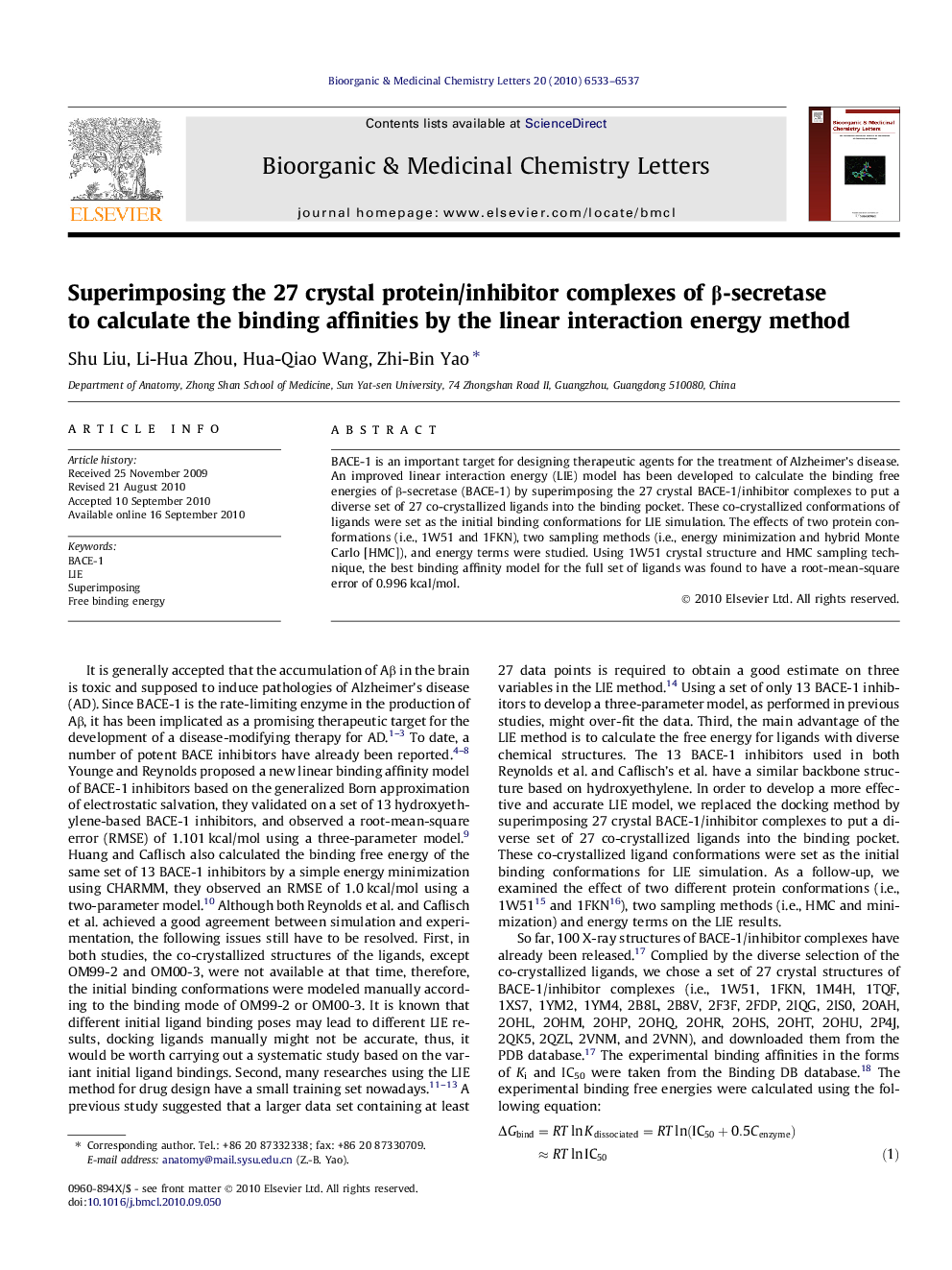
BACE-1 is an important target for designing therapeutic agents for the treatment of Alzheimer’s disease. An improved linear interaction energy (LIE) model has been developed to calculate the binding free energies of β-secretase (BACE-1) by superimposing the 27 crystal BACE-1/inhibitor complexes to put a diverse set of 27 co-crystallized ligands into the binding pocket. These co-crystallized conformations of ligands were set as the initial binding conformations for LIE simulation. The effects of two protein conformations (i.e., 1W51 and 1FKN), two sampling methods (i.e., energy minimization and hybrid Monte Carlo [HMC]), and energy terms were studied. Using 1W51 crystal structure and HMC sampling technique, the best binding affinity model for the full set of ligands was found to have a root-mean-square error of 0.996 kcal/mol.
An improved linear interaction energy (LIE) model has been developed to calculate the binding free energies of β-secretase (BACE-1), a diverse set of 27 co-crystallized ligands were put into the binding pocket by superimposing the 27 crystal BACE-1/inhibitor complexes.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 20, Issue 22, 15 November 2010, Pages 6533–6537