| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1372159 | 981866 | 2011 | 4 صفحه PDF | دانلود رایگان |
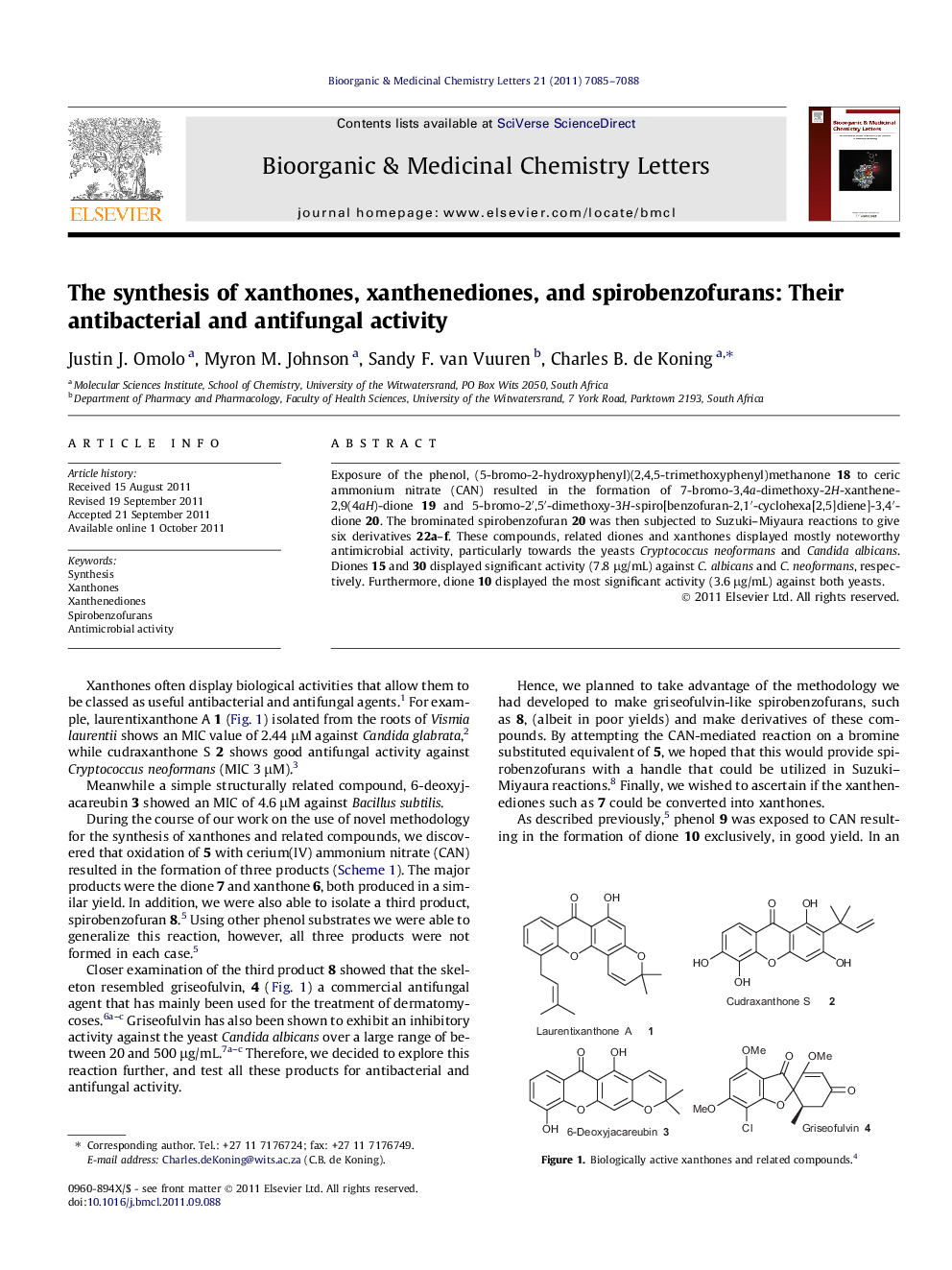
Exposure of the phenol, (5-bromo-2-hydroxyphenyl)(2,4,5-trimethoxyphenyl)methanone 18 to ceric ammonium nitrate (CAN) resulted in the formation of 7-bromo-3,4a-dimethoxy-2H-xanthene-2,9(4aH)-dione 19 and 5-bromo-2′,5′-dimethoxy-3H-spiro[benzofuran-2,1′-cyclohexa[2,5]diene]-3,4′-dione 20. The brominated spirobenzofuran 20 was then subjected to Suzuki–Miyaura reactions to give six derivatives 22a–f. These compounds, related diones and xanthones displayed mostly noteworthy antimicrobial activity, particularly towards the yeasts Cryptococcus neoformans and Candida albicans. Diones 15 and 30 displayed significant activity (7.8 μg/mL) against C. albicans and C. neoformans, respectively. Furthermore, dione 10 displayed the most significant activity (3.6 μg/mL) against both yeasts.
A range of spirobenzofurans and related compounds were synthesized and tested for their antibacterial and antifungal activity. Some displayed significant activity against the yeasts Cryptococcus neoformans and Candida albicans.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 21, Issue 23, 1 December 2011, Pages 7085–7088