| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1376043 | 981949 | 2007 | 5 صفحه PDF | دانلود رایگان |
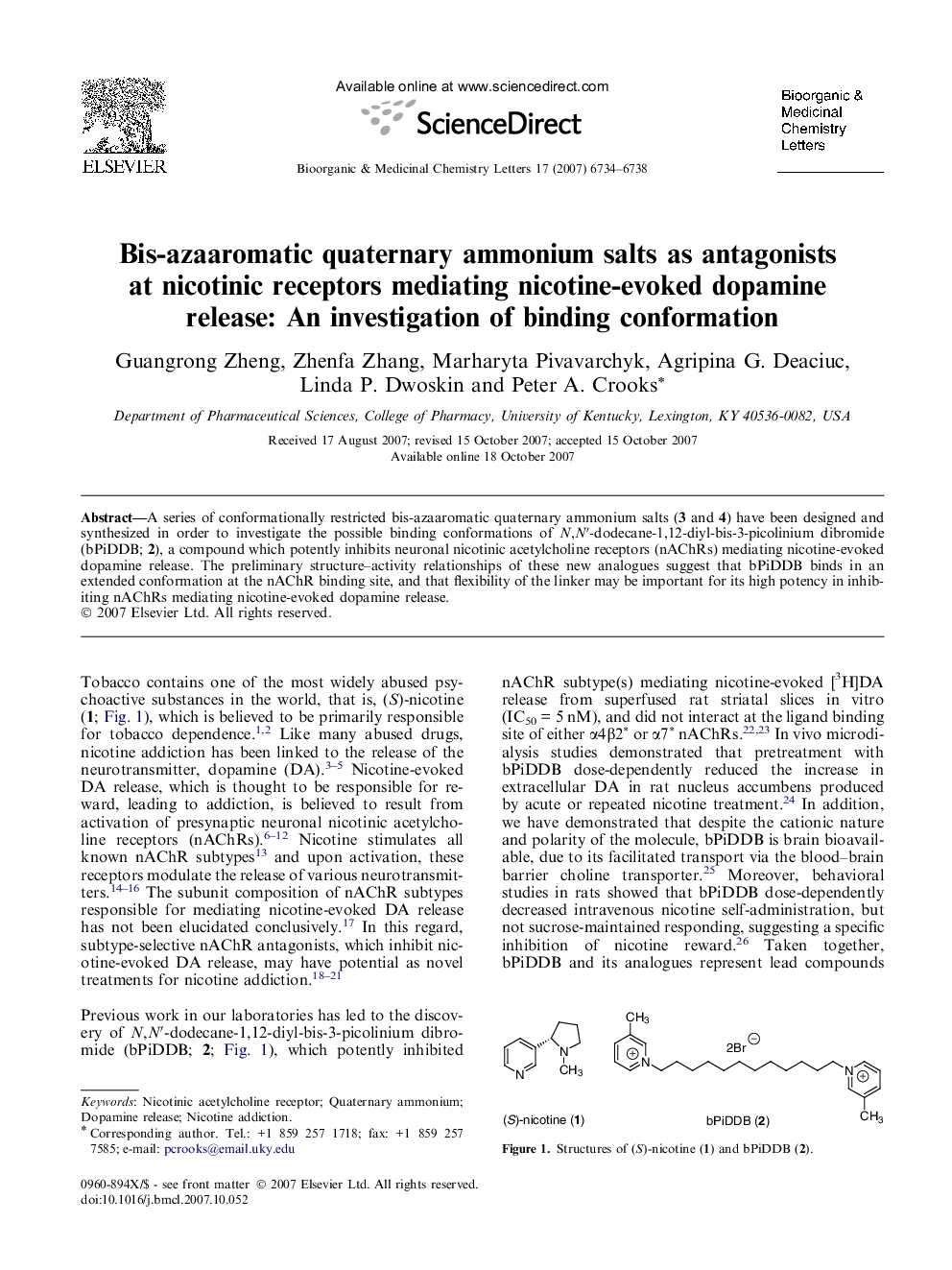
A series of conformationally restricted bis-azaaromatic quaternary ammonium salts (3 and 4) have been designed and synthesized in order to investigate the possible binding conformations of N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; 2), a compound which potently inhibits neuronal nicotinic acetylcholine receptors (nAChRs) mediating nicotine-evoked dopamine release. The preliminary structure–activity relationships of these new analogues suggest that bPiDDB binds in an extended conformation at the nAChR binding site, and that flexibility of the linker may be important for its high potency in inhibiting nAChRs mediating nicotine-evoked dopamine release.
A series of conformationally restricted bis-azaaromatic quaternary ammonium salts (3 and 4) have been designed and synthesized in order to investigate the possible binding conformations of N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB; 2), a compound which potently inhibits neuronal nicotinic acetylcholine receptors (nAChRs) mediating nicotine-evoked dopamine release. The preliminary structure–activity relationships of these new analogues suggest that bPiDDB binds in an extended conformation at the nAChR binding site, and that flexibility of the linker is important for its high potency in inhibiting nAChRs mediating nicotine-evoked dopamine release.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 17, Issue 24, 15 December 2007, Pages 6734–6738