| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1377980 | 981992 | 2007 | 5 صفحه PDF | دانلود رایگان |
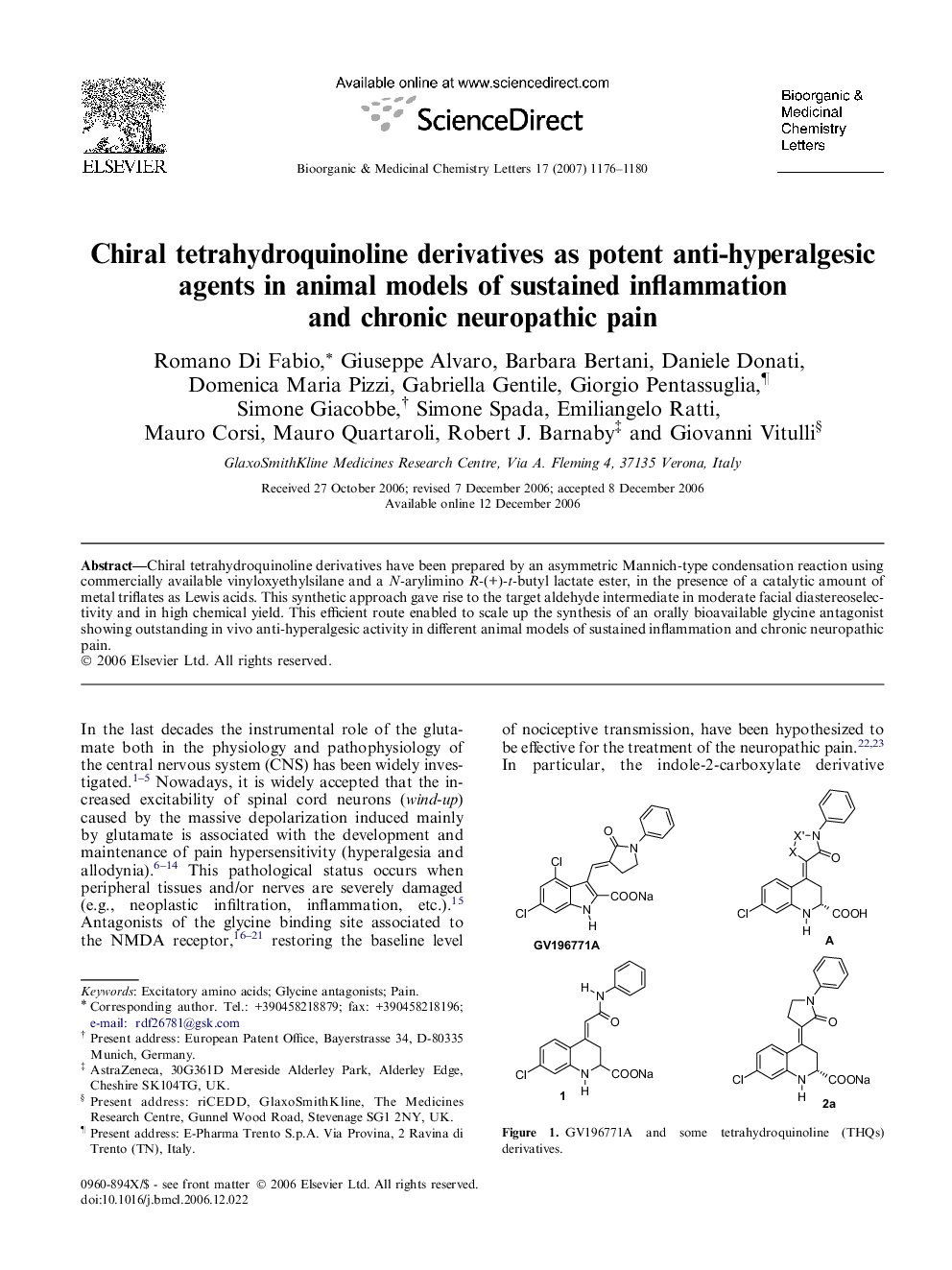
Chiral tetrahydroquinoline derivatives have been prepared by an asymmetric Mannich-type condensation reaction using commercially available vinyloxyethylsilane and a N-arylimino R-(+)-t-butyl lactate ester, in the presence of a catalytic amount of metal triflates as Lewis acids. This synthetic approach gave rise to the target aldehyde intermediate in moderate facial diastereoselectivity and in high chemical yield. This efficient route enabled to scale up the synthesis of an orally bioavailable glycine antagonist showing outstanding in vivo anti-hyperalgesic activity in different animal models of sustained inflammation and chronic neuropathic pain.
Chiral tetrahydroquinolines have been prepared by an asymmetric Mannich-type condensation reaction as in vivo potent anti-hyperalgesic agents in different animal models of chronic pain.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry Letters - Volume 17, Issue 5, 1 March 2007, Pages 1176–1180