| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 2035067 | 1072129 | 2016 | 13 صفحه PDF | دانلود رایگان |
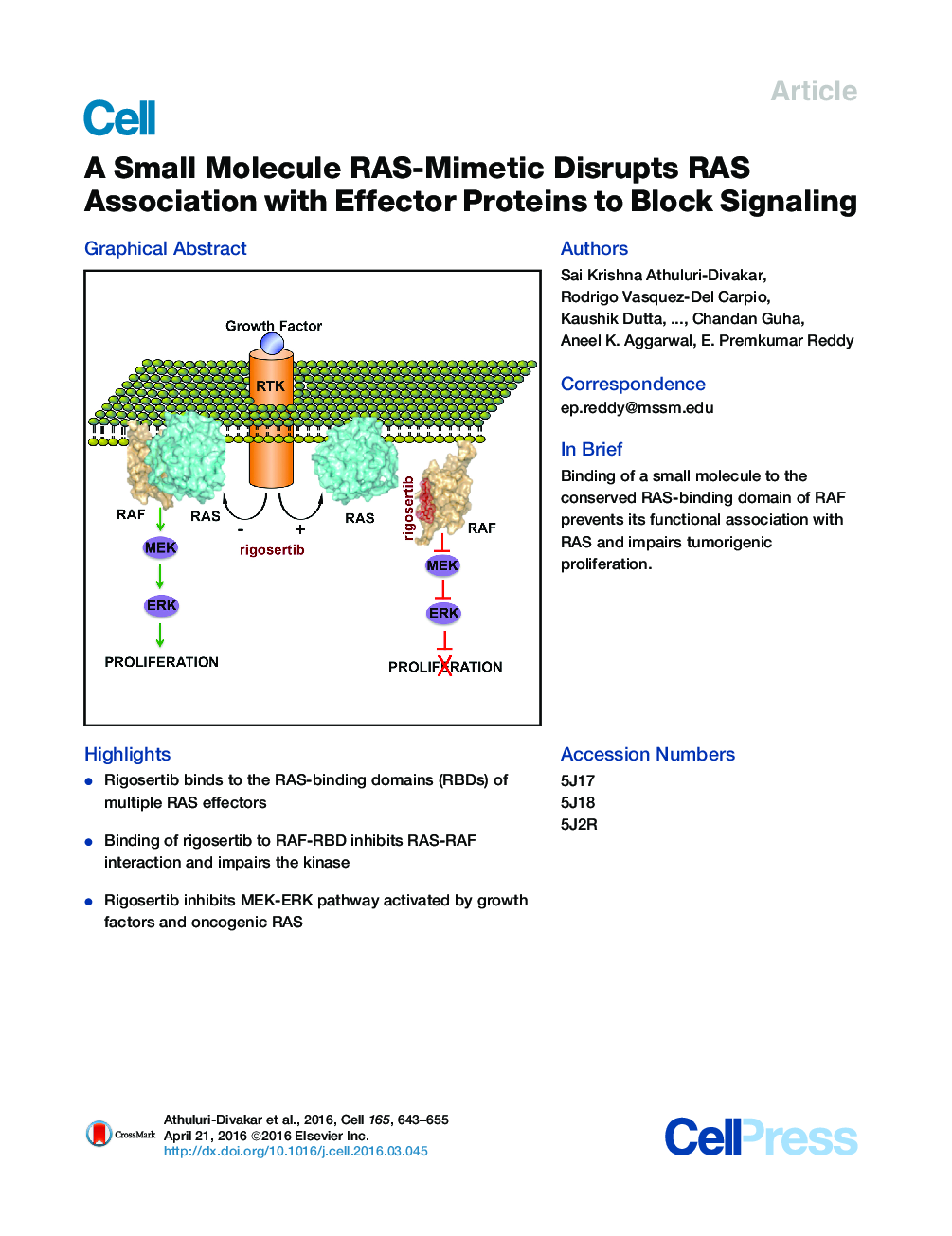
• Rigosertib binds to the RAS-binding domains (RBDs) of multiple RAS effectors
• Binding of rigosertib to RAF-RBD inhibits RAS-RAF interaction and impairs the kinase
• Rigosertib inhibits MEK-ERK pathway activated by growth factors and oncogenic RAS
SummaryOncogenic activation of RAS genes via point mutations occurs in 20%–30% of human cancers. The development of effective RAS inhibitors has been challenging, necessitating new approaches to inhibit this oncogenic protein. Functional studies have shown that the switch region of RAS interacts with a large number of effector proteins containing a common RAS-binding domain (RBD). Because RBD-mediated interactions are essential for RAS signaling, blocking RBD association with small molecules constitutes an attractive therapeutic approach. Here, we present evidence that rigosertib, a styryl-benzyl sulfone, acts as a RAS-mimetic and interacts with the RBDs of RAF kinases, resulting in their inability to bind to RAS, disruption of RAF activation, and inhibition of the RAS-RAF-MEK pathway. We also find that rigosertib binds to the RBDs of Ral-GDS and PI3Ks. These results suggest that targeting of RBDs across multiple signaling pathways by rigosertib may represent an effective strategy for inactivation of RAS signaling.
Graphical AbstractFigure optionsDownload high-quality image (286 K)Download as PowerPoint slide
Journal: - Volume 165, Issue 3, 21 April 2016, Pages 643–655