| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 205278 | 461102 | 2016 | 10 صفحه PDF | دانلود رایگان |
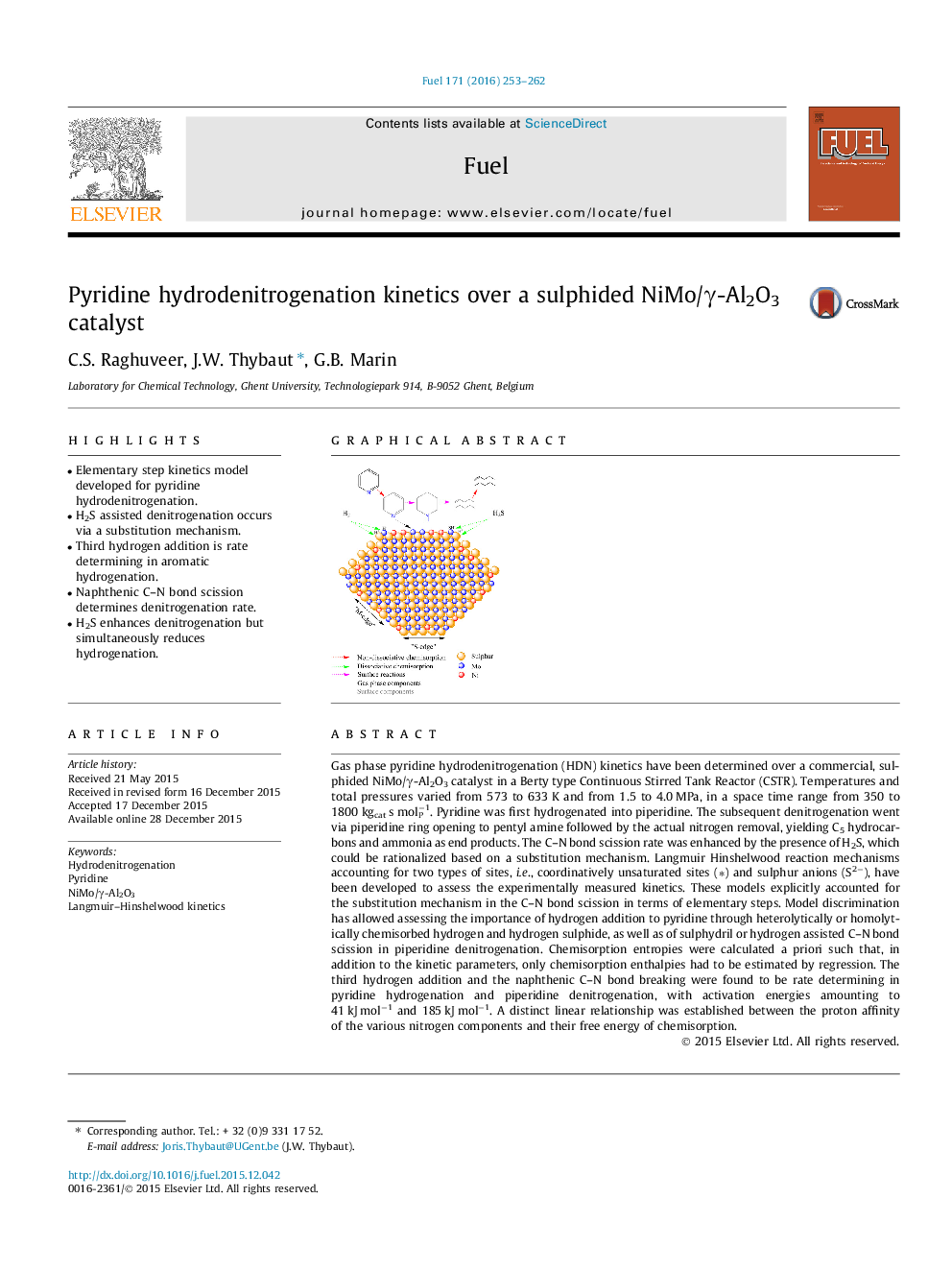
• Elementary step kinetics model developed for pyridine hydrodenitrogenation.
• H2S assisted denitrogenation occurs via a substitution mechanism.
• Third hydrogen addition is rate determining in aromatic hydrogenation.
• Naphthenic C–N bond scission determines denitrogenation rate.
• H2S enhances denitrogenation but simultaneously reduces hydrogenation.
Gas phase pyridine hydrodenitrogenation (HDN) kinetics have been determined over a commercial, sulphided NiMo/γ-Al2O3 catalyst in a Berty type Continuous Stirred Tank Reactor (CSTR). Temperatures and total pressures varied from 573 to 633 K and from 1.5 to 4.0 MPa, in a space time range from 350 to 1800 kgcat s mol−1P. Pyridine was first hydrogenated into piperidine. The subsequent denitrogenation went via piperidine ring opening to pentyl amine followed by the actual nitrogen removal, yielding C5 hydrocarbons and ammonia as end products. The C–N bond scission rate was enhanced by the presence of H2S, which could be rationalized based on a substitution mechanism. Langmuir Hinshelwood reaction mechanisms accounting for two types of sites, i.e., coordinatively unsaturated sites (∗) and sulphur anions (S2−), have been developed to assess the experimentally measured kinetics. These models explicitly accounted for the substitution mechanism in the C–N bond scission in terms of elementary steps. Model discrimination has allowed assessing the importance of hydrogen addition to pyridine through heterolytically or homolytically chemisorbed hydrogen and hydrogen sulphide, as well as of sulphydril or hydrogen assisted C–N bond scission in piperidine denitrogenation. Chemisorption entropies were calculated a priori such that, in addition to the kinetic parameters, only chemisorption enthalpies had to be estimated by regression. The third hydrogen addition and the naphthenic C–N bond breaking were found to be rate determining in pyridine hydrogenation and piperidine denitrogenation, with activation energies amounting to 41 kJ mol−1 and 185 kJ mol−1. A distinct linear relationship was established between the proton affinity of the various nitrogen components and their free energy of chemisorption.
Figure optionsDownload as PowerPoint slide
Journal: Fuel - Volume 171, 1 May 2016, Pages 253–262