| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 205923 | 461128 | 2015 | 8 صفحه PDF | دانلود رایگان |
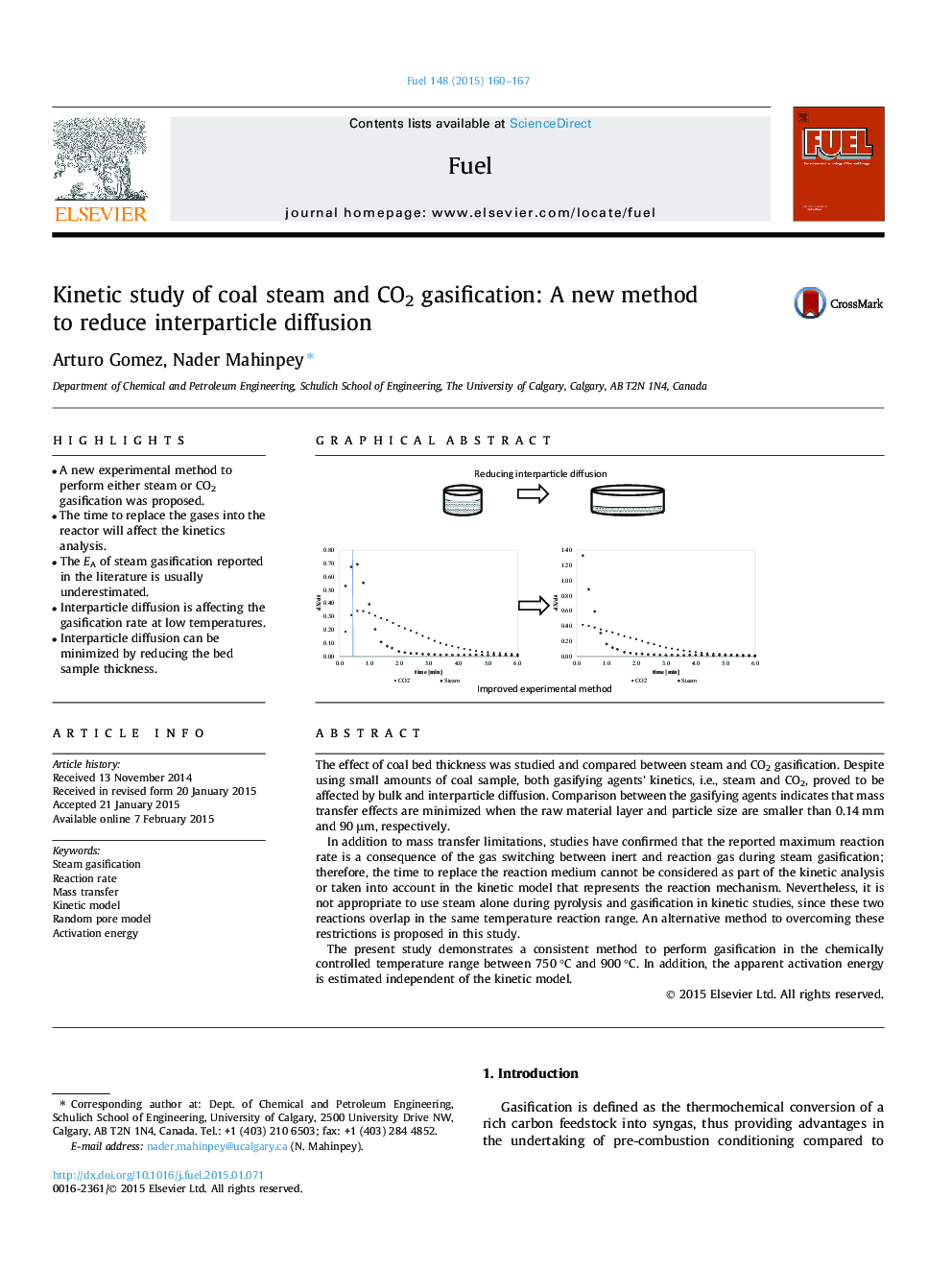
• A new experimental method to perform either steam or CO2 gasification was proposed.
• The time to replace the gases into the reactor will affect the kinetics analysis.
• The EA of steam gasification reported in the literature is usually underestimated.
• Interparticle diffusion is affecting the gasification rate at low temperatures.
• Interparticle diffusion can be minimized by reducing the bed sample thickness.
The effect of coal bed thickness was studied and compared between steam and CO2 gasification. Despite using small amounts of coal sample, both gasifying agents’ kinetics, i.e., steam and CO2, proved to be affected by bulk and interparticle diffusion. Comparison between the gasifying agents indicates that mass transfer effects are minimized when the raw material layer and particle size are smaller than 0.14 mm and 90 μm, respectively.In addition to mass transfer limitations, studies have confirmed that the reported maximum reaction rate is a consequence of the gas switching between inert and reaction gas during steam gasification; therefore, the time to replace the reaction medium cannot be considered as part of the kinetic analysis or taken into account in the kinetic model that represents the reaction mechanism. Nevertheless, it is not appropriate to use steam alone during pyrolysis and gasification in kinetic studies, since these two reactions overlap in the same temperature reaction range. An alternative method to overcoming these restrictions is proposed in this study.The present study demonstrates a consistent method to perform gasification in the chemically controlled temperature range between 750 °C and 900 °C. In addition, the apparent activation energy is estimated independent of the kinetic model.
Figure optionsDownload as PowerPoint slide
Journal: Fuel - Volume 148, 15 May 2015, Pages 160–167