| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 443425 | 692722 | 2013 | 12 صفحه PDF | دانلود رایگان |
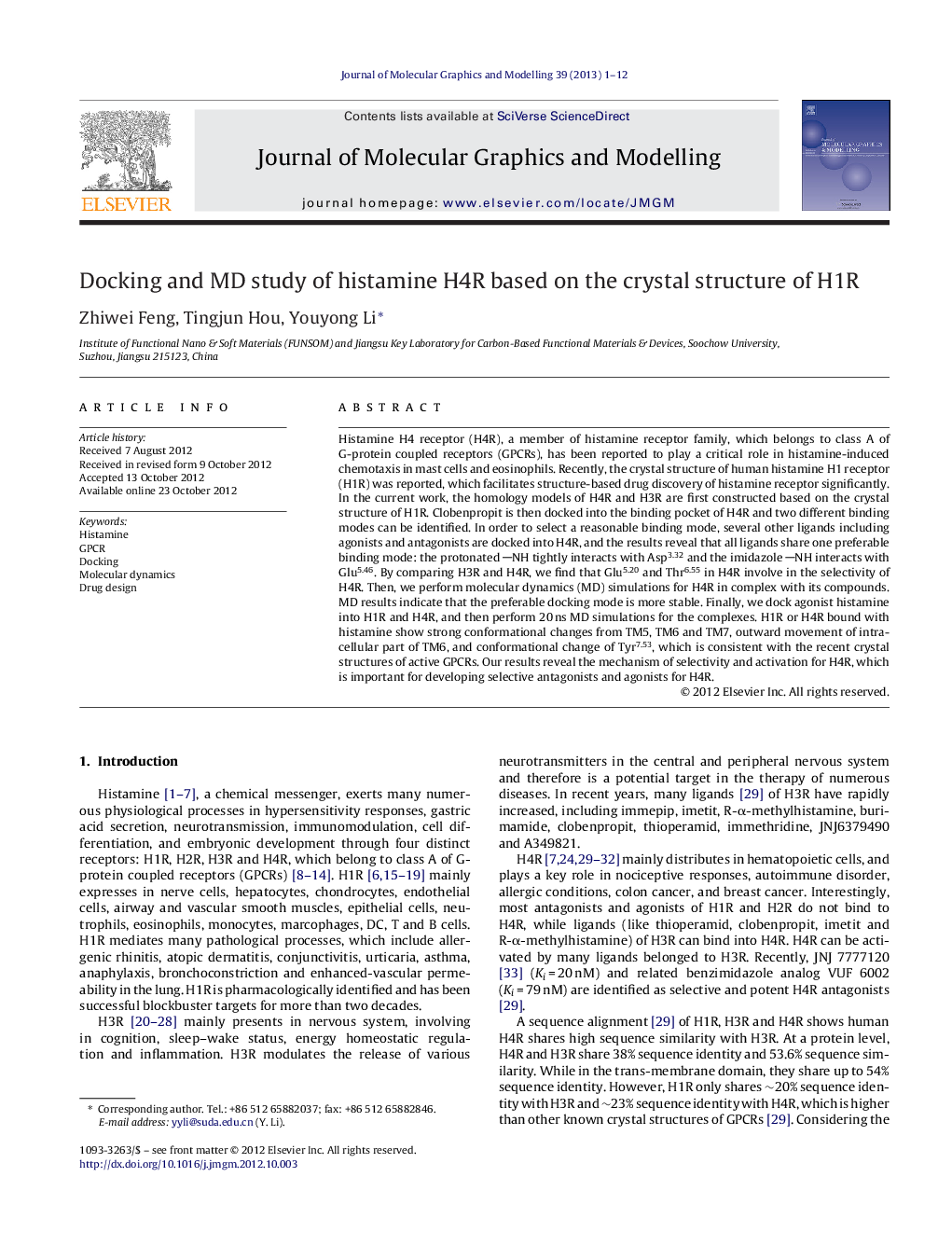
Histamine H4 receptor (H4R), a member of histamine receptor family, which belongs to class A of G-protein coupled receptors (GPCRs), has been reported to play a critical role in histamine-induced chemotaxis in mast cells and eosinophils. Recently, the crystal structure of human histamine H1 receptor (H1R) was reported, which facilitates structure-based drug discovery of histamine receptor significantly. In the current work, the homology models of H4R and H3R are first constructed based on the crystal structure of H1R. Clobenpropit is then docked into the binding pocket of H4R and two different binding modes can be identified. In order to select a reasonable binding mode, several other ligands including agonists and antagonists are docked into H4R, and the results reveal that all ligands share one preferable binding mode: the protonated NH tightly interacts with Asp3.32 and the imidazole NH interacts with Glu5.46. By comparing H3R and H4R, we find that Glu5.20 and Thr6.55 in H4R involve in the selectivity of H4R. Then, we perform molecular dynamics (MD) simulations for H4R in complex with its compounds. MD results indicate that the preferable docking mode is more stable. Finally, we dock agonist histamine into H1R and H4R, and then perform 20 ns MD simulations for the complexes. H1R or H4R bound with histamine show strong conformational changes from TM5, TM6 and TM7, outward movement of intracellular part of TM6, and conformational change of Tyr7.53, which is consistent with the recent crystal structures of active GPCRs. Our results reveal the mechanism of selectivity and activation for H4R, which is important for developing selective antagonists and agonists for H4R.
20 ns molecular dynamics is performed for H4R with its agonist and antagonist with lipid and water. (a) By comparing H3R and H4R, we find that Glu5.20 and Thr6.55 in H4R involve in the selectivity of H4R. (b) H4R bound with histamine (in magenta color) shows strong conformational changes from TM5, TM6, and TM7, outward movement of intracellular part of TM6, and conformational change of Tyr7.53, which is consistent with the recent crystal structures (in yellow color) of active GPCRs. Our results reveal the mechanism of selectivity and activation for H4R, which is important for developing selective antagonists and agonists for H4R. The arrow indicates movement of TM5, TM6, and TM7 in H4R with agonist bound.Figure optionsDownload high-quality image (348 K)Download as PowerPoint slideHighlights
► Based on recent H1R crystal structure, we develop homology models of H4R/H3R.
► We perform MD study of H1R, H3R and H4R bound with ligands in lipid and water.
► We identify the favorable binding mode and important residues involved in binding.
► Our results reveal the important residues for selectivity for H4R.
► Our MD results reveal the conformational changes of H1R and H4R.
Journal: Journal of Molecular Graphics and Modelling - Volume 39, February 2013, Pages 1–12