| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 606232 | 1454517 | 2016 | 8 صفحه PDF | دانلود رایگان |
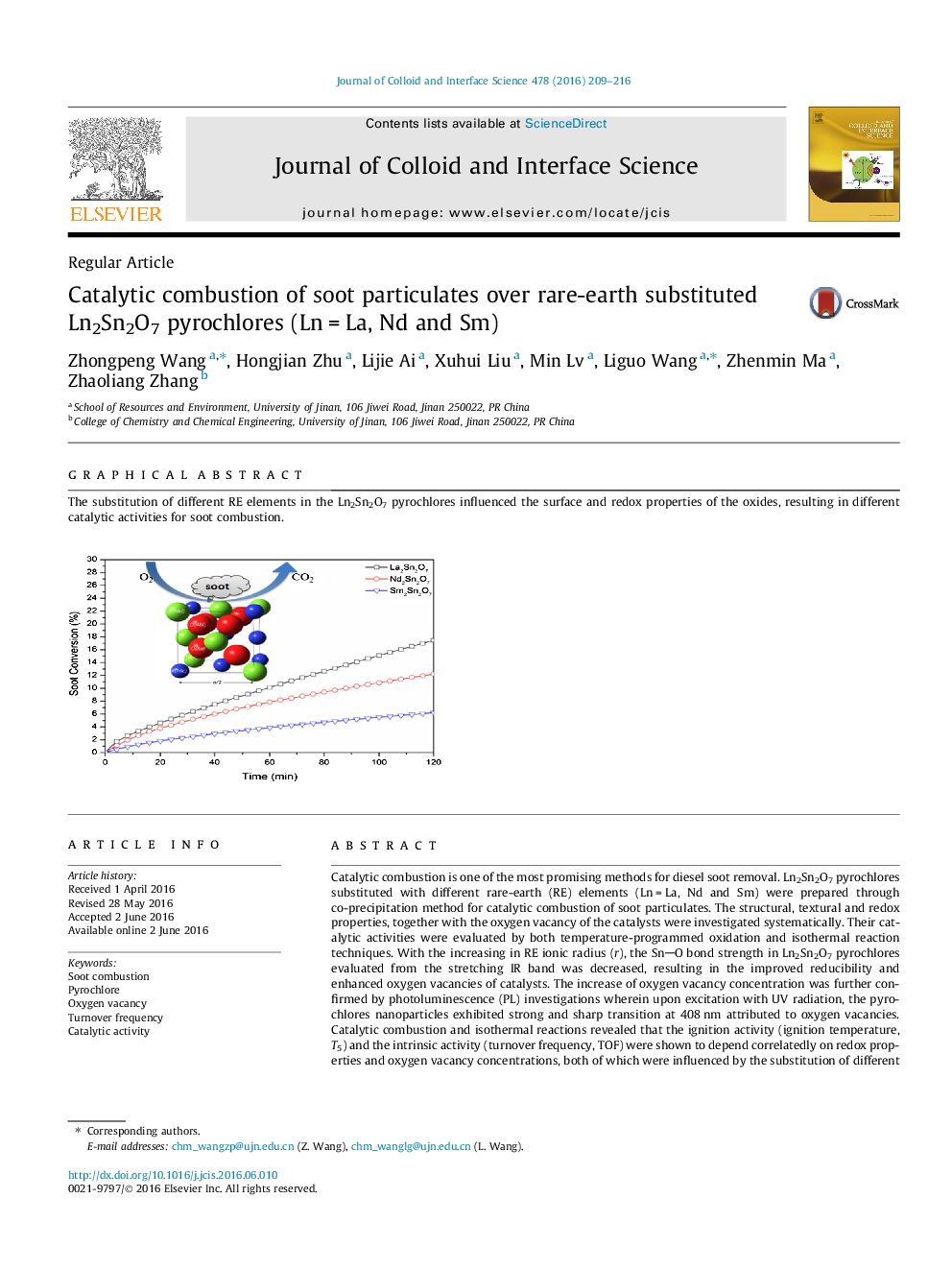
Catalytic combustion is one of the most promising methods for diesel soot removal. Ln2Sn2O7 pyrochlores substituted with different rare-earth (RE) elements (Ln = La, Nd and Sm) were prepared through co-precipitation method for catalytic combustion of soot particulates. The structural, textural and redox properties, together with the oxygen vacancy of the catalysts were investigated systematically. Their catalytic activities were evaluated by both temperature-programmed oxidation and isothermal reaction techniques. With the increasing in RE ionic radius (r), the SnO bond strength in Ln2Sn2O7 pyrochlores evaluated from the stretching IR band was decreased, resulting in the improved reducibility and enhanced oxygen vacancies of catalysts. The increase of oxygen vacancy concentration was further confirmed by photoluminescence (PL) investigations wherein upon excitation with UV radiation, the pyrochlores nanoparticles exhibited strong and sharp transition at 408 nm attributed to oxygen vacancies. Catalytic combustion and isothermal reactions revealed that the ignition activity (ignition temperature, T5) and the intrinsic activity (turnover frequency, TOF) were shown to depend correlatedly on redox properties and oxygen vacancy concentrations, both of which were influenced by the substitution of different RE elements. Among the pyrochlore oxides, the as-synthesized La2Sn2O7 sample displayed relatively the highest ignition activity and the largest intrinsic activity with TOF of 2.33 × 10−3 s−1.
The substitution of different RE elements in the Ln2Sn2O7 pyrochlores influenced the surface and redox properties of the oxides, resulting in different catalytic activities for soot combustion.Figure optionsDownload high-quality image (119 K)Download as PowerPoint slide
Journal: Journal of Colloid and Interface Science - Volume 478, 15 September 2016, Pages 209–216