| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 61425 | 47581 | 2013 | 11 صفحه PDF | دانلود رایگان |
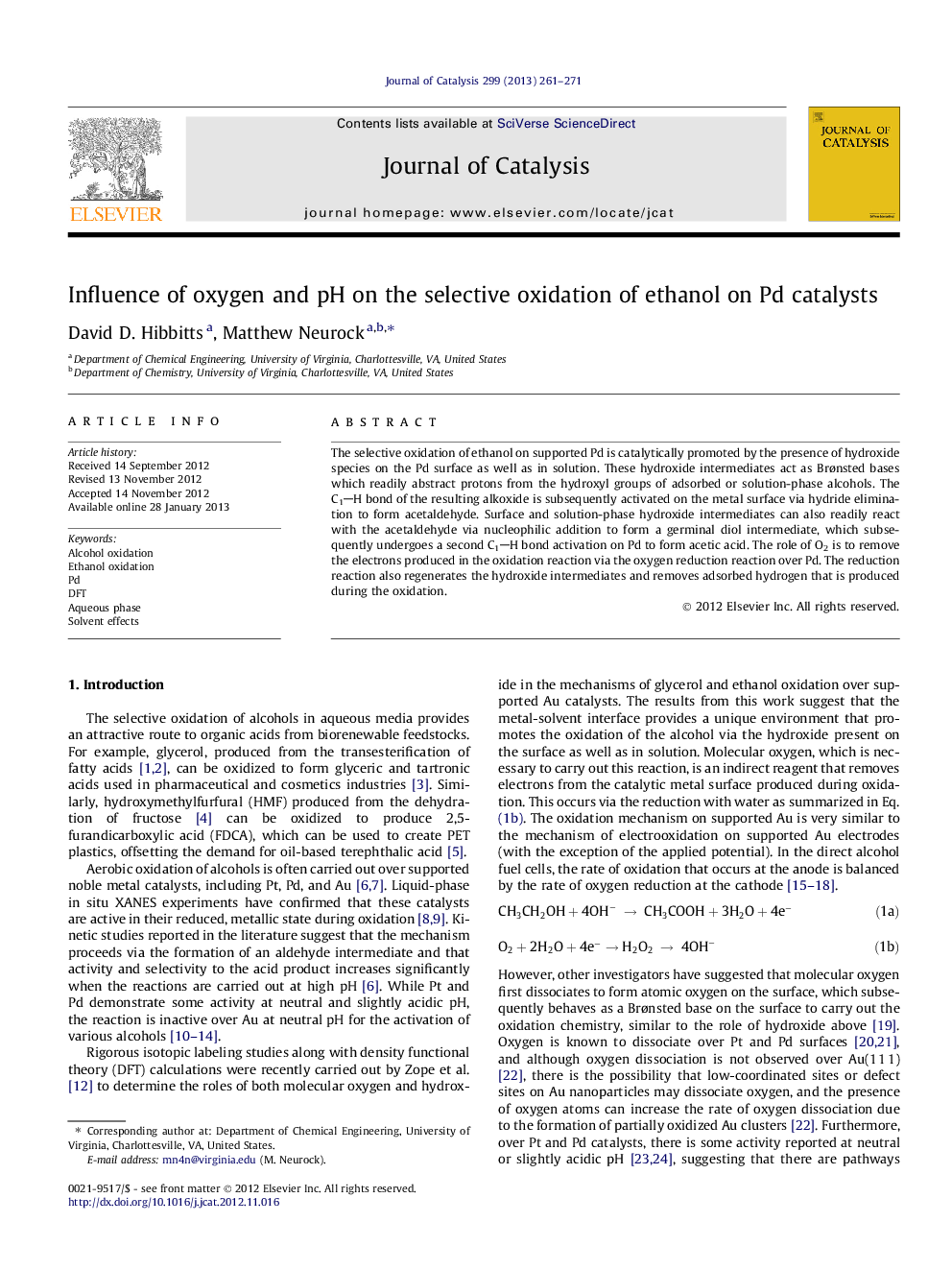
The selective oxidation of ethanol on supported Pd is catalytically promoted by the presence of hydroxide species on the Pd surface as well as in solution. These hydroxide intermediates act as Brønsted bases which readily abstract protons from the hydroxyl groups of adsorbed or solution-phase alcohols. The C1H bond of the resulting alkoxide is subsequently activated on the metal surface via hydride elimination to form acetaldehyde. Surface and solution-phase hydroxide intermediates can also readily react with the acetaldehyde via nucleophilic addition to form a germinal diol intermediate, which subsequently undergoes a second C1H bond activation on Pd to form acetic acid. The role of O2 is to remove the electrons produced in the oxidation reaction via the oxygen reduction reaction over Pd. The reduction reaction also regenerates the hydroxide intermediates and removes adsorbed hydrogen that is produced during the oxidation.
The selective oxidation of ethanol on supported Pd is catalytically promoted by the presence of hydroxide species on the Pd surface as well as in solution. These hydroxide intermediates act as Brønsted bases which readily abstract protons from the hydroxyl groups of adsorbed or solution-phase alcohols and are critical during the oxygen-addition step to form the acid products. At neutral/low pH conditions, the scarcity of hydroxide results in metal-catalyzed dehydrogenation of the alcohol, lowering the reaction rate.Figure optionsDownload high-quality image (154 K)Download as PowerPoint slideHighlights
► Selective oxidation of alcohols over Pd is controlled by oxygen and solution pH.
► Hydroxide, on Pd and in solution, acts as a base to catalyze dehydrogenation.
► Hydroxide nucleophilically attacks the aldehyde intermediate to form the acid.
► Oxygen is reduced through direct reactions with water to form hydroxide and water.
► At low pH, Pd-catalyzed dehydrogenation is rate-limiting due to the lack of hydroxide ions.
Journal: Journal of Catalysis - Volume 299, March 2013, Pages 261–271