| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 64495 | 48357 | 2006 | 10 صفحه PDF | دانلود رایگان |
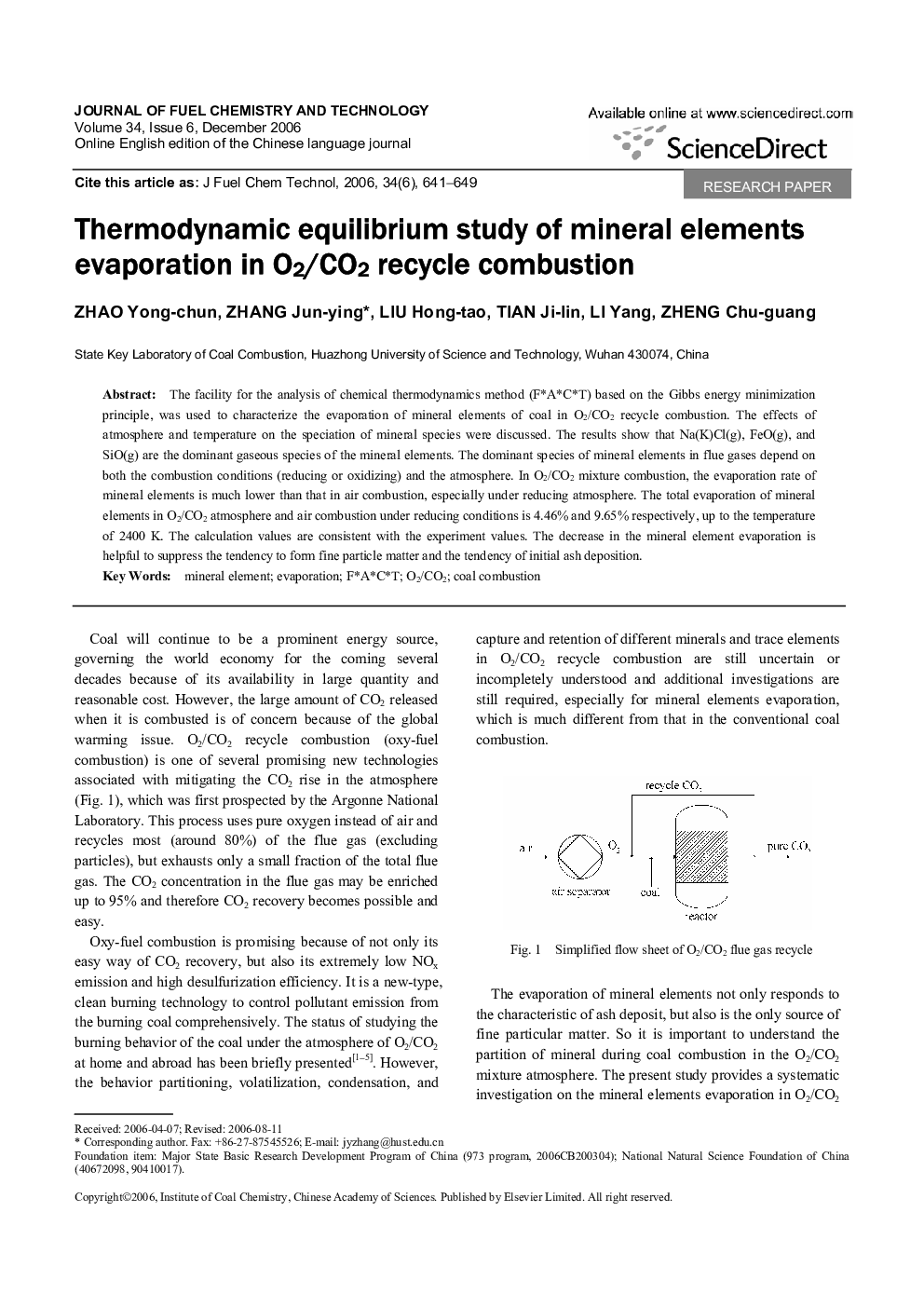
The facility for the analysis of chemical thermodynamics method (F*A*C*T) based on the Gibbs energy minimization principle, was used to characterize the evaporation of mineral elements of coal in O2/CO2 recycle combustion. The effects of atmosphere and temperature on the speciation of mineral species were discussed. The results show that Na(K)Cl(g), FeO(g), and SiO(g) are the dominant gaseous species of the mineral elements. The dominant species of mineral elements in flue gases depend on both the combustion conditions (reducing or oxidizing) and the atmosphere. In O2/CO2 mixture combustion, the evaporation rate of mineral elements is much lower than that in air combustion, especially under reducing atmosphere. The total evaporation of mineral elements in O2/CO2 atmosphere and air combustion under reducing conditions is 4.46% and 9.65% respectively, up to the temperature of 2400 K. The calculation values are consistent with the experiment values. The decrease in the mineral element evaporation is helpful to suppress the tendency to form fine particle matter and the tendency of initial ash deposition.
Journal: Journal of Fuel Chemistry and Technology - Volume 34, Issue 6, December 2006, Pages 641-650