| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6475347 | 1424969 | 2017 | 9 صفحه PDF | دانلود رایگان |
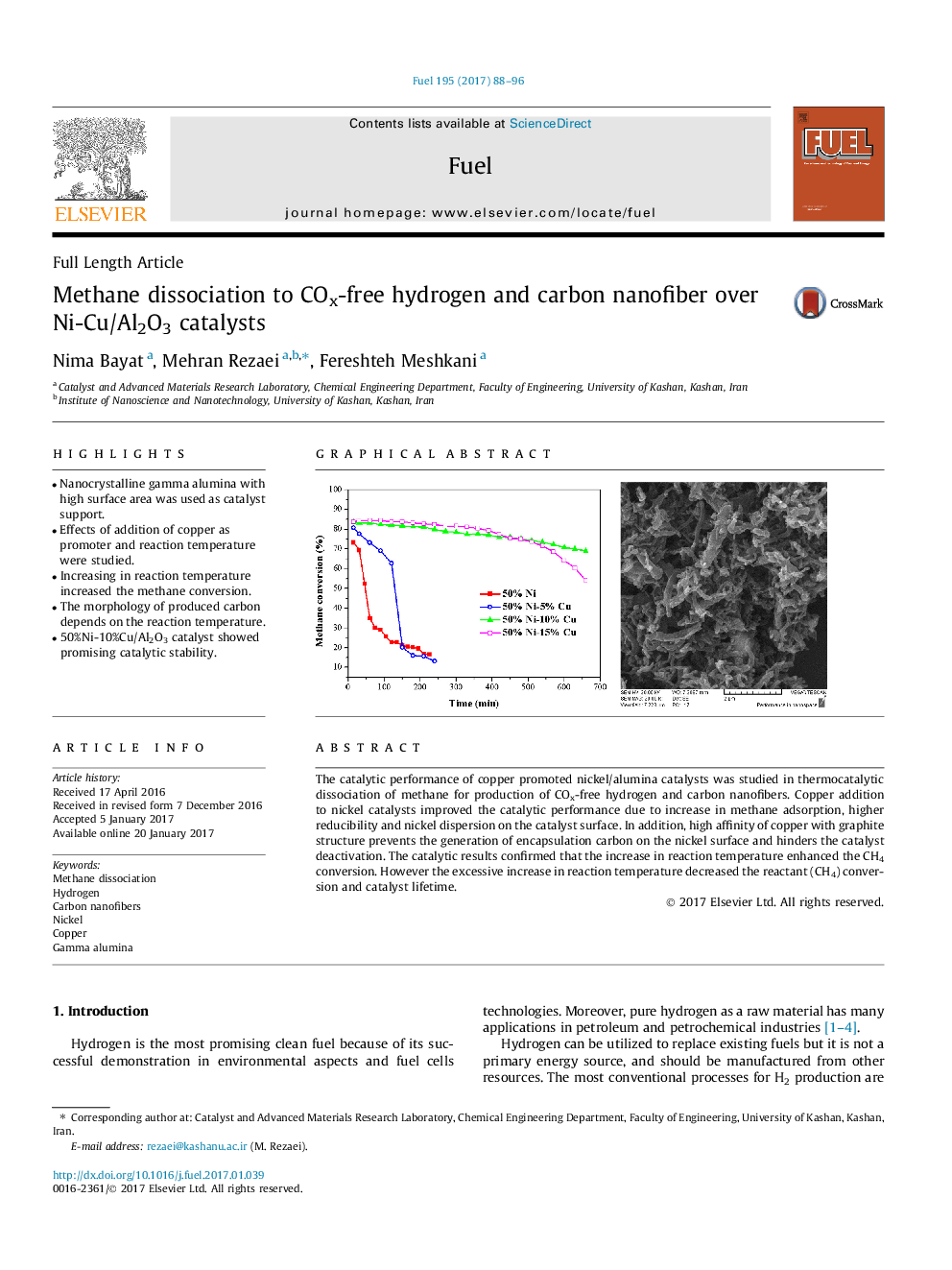
• Nanocrystalline gamma alumina with high surface area was used as catalyst support.
• Effects of addition of copper as promoter and reaction temperature were studied.
• Increasing in reaction temperature increased the methane conversion.
• The morphology of produced carbon depends on the reaction temperature.
• 50%Ni-10%Cu/Al2O3 catalyst showed promising catalytic stability.
The catalytic performance of copper promoted nickel/alumina catalysts was studied in thermocatalytic dissociation of methane for production of COx-free hydrogen and carbon nanofibers. Copper addition to nickel catalysts improved the catalytic performance due to increase in methane adsorption, higher reducibility and nickel dispersion on the catalyst surface. In addition, high affinity of copper with graphite structure prevents the generation of encapsulation carbon on the nickel surface and hinders the catalyst deactivation. The catalytic results confirmed that the increase in reaction temperature enhanced the CH4 conversion. However the excessive increase in reaction temperature decreased the reactant (CH4) conversion and catalyst lifetime.
Addition of copper to nickel catalyst supported on mesoporous nanocrystalline gamma alumina improved the catalytic activity and stability in thermocatalytic decomposition of methane. SEM micrograph of the spent catalyst (50%Ni-10%Cu/Al2O3 at 675 °C) exhibited the formation of filamentous carbon.Figure optionsDownload high-quality image (123 K)Download as PowerPoint slide
Journal: Fuel - Volume 195, 1 May 2017, Pages 88–96