| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6476016 | 1424978 | 2016 | 10 صفحه PDF | دانلود رایگان |
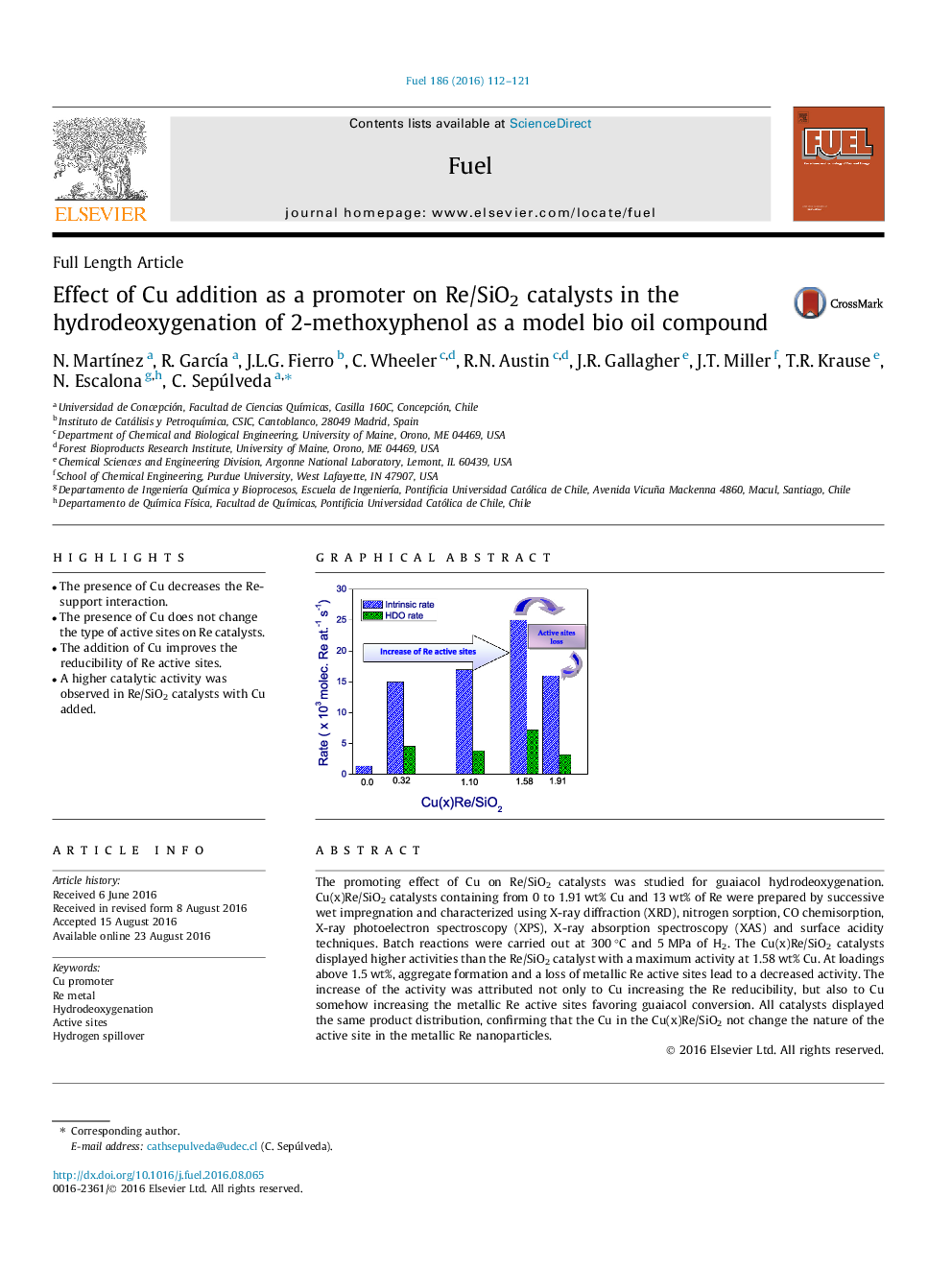
• The presence of Cu decreases the Re-support interaction.
• The presence of Cu does not change the type of active sites on Re catalysts.
• The addition of Cu improves the reducibility of Re active sites.
• A higher catalytic activity was observed in Re/SiO2 catalysts with Cu added.
The promoting effect of Cu on Re/SiO2 catalysts was studied for guaiacol hydrodeoxygenation. Cu(x)Re/SiO2 catalysts containing from 0 to 1.91 wt% Cu and 13 wt% of Re were prepared by successive wet impregnation and characterized using X-ray diffraction (XRD), nitrogen sorption, CO chemisorption, X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (XAS) and surface acidity techniques. Batch reactions were carried out at 300 °C and 5 MPa of H2. The Cu(x)Re/SiO2 catalysts displayed higher activities than the Re/SiO2 catalyst with a maximum activity at 1.58 wt% Cu. At loadings above 1.5 wt%, aggregate formation and a loss of metallic Re active sites lead to a decreased activity. The increase of the activity was attributed not only to Cu increasing the Re reducibility, but also to Cu somehow increasing the metallic Re active sites favoring guaiacol conversion. All catalysts displayed the same product distribution, confirming that the Cu in the Cu(x)Re/SiO2 not change the nature of the active site in the metallic Re nanoparticles.
Graphical AbstractFigure optionsDownload high-quality image (208 K)Download as PowerPoint slide
Journal: Fuel - Volume 186, 15 December 2016, Pages 112–121