| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 10605629 | 982784 | 2011 | 7 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
Reactivity switching and selective activation of C-1 or C-3 in 2,3-unsaturated thioglycosides
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
کلمات کلیدی
موضوعات مرتبط
مهندسی و علوم پایه
شیمی
شیمی آلی
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
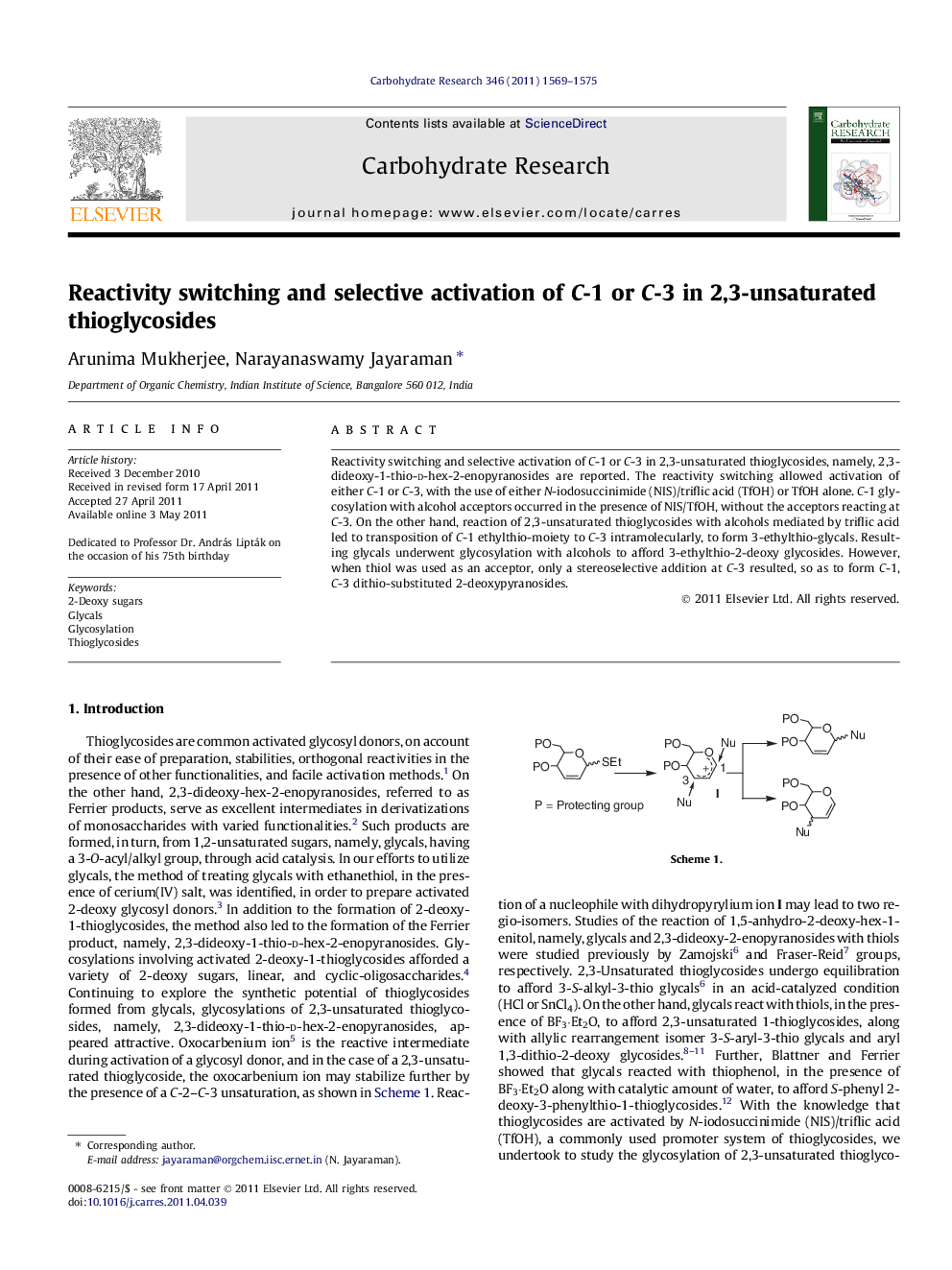
Reactivity switching and selective activation of C-1 or C-3 in 2,3-unsaturated thioglycosides, namely, 2,3-dideoxy-1-thio-d-hex-2-enopyranosides are reported. The reactivity switching allowed activation of either C-1 or C-3, with the use of either N-iodosuccinimide (NIS)/triflic acid (TfOH) or TfOH alone. C-1 glycosylation with alcohol acceptors occurred in the presence of NIS/TfOH, without the acceptors reacting at C-3. On the other hand, reaction of 2,3-unsaturated thioglycosides with alcohols mediated by triflic acid led to transposition of C-1 ethylthio-moiety to C-3 intramolecularly, to form 3-ethylthio-glycals. Resulting glycals underwent glycosylation with alcohols to afford 3-ethylthio-2-deoxy glycosides. However, when thiol was used as an acceptor, only a stereoselective addition at C-3 resulted, so as to form C-1, C-3 dithio-substituted 2-deoxypyranosides.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Carbohydrate Research - Volume 346, Issue 12, 6 September 2011, Pages 1569-1575
Journal: Carbohydrate Research - Volume 346, Issue 12, 6 September 2011, Pages 1569-1575
نویسندگان
Arunima Mukherjee, Narayanaswamy Jayaraman,