| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229041 | 1495231 | 2015 | 6 صفحه PDF | دانلود رایگان |
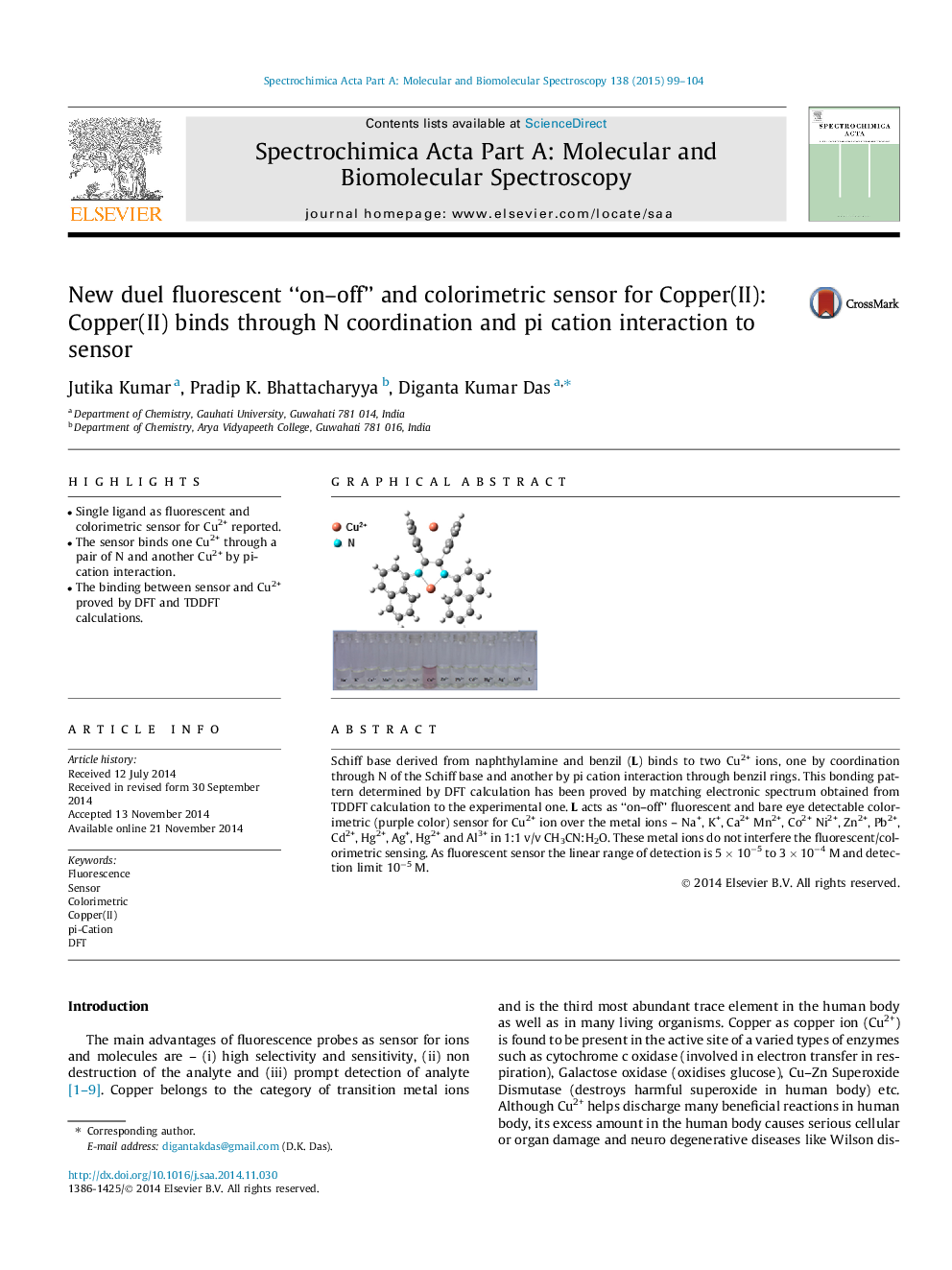
• Single ligand as fluorescent and colorimetric sensor for Cu2+ reported.
• The sensor binds one Cu2+ through a pair of N and another Cu2+ by pi-cation interaction.
• The binding between sensor and Cu2+ proved by DFT and TDDFT calculations.
Schiff base derived from naphthylamine and benzil (L) binds to two Cu2+ ions, one by coordination through N of the Schiff base and another by pi cation interaction through benzil rings. This bonding pattern determined by DFT calculation has been proved by matching electronic spectrum obtained from TDDFT calculation to the experimental one. L acts as “on–off” fluorescent and bare eye detectable colorimetric (purple color) sensor for Cu2+ ion over the metal ions – Na+, K+, Ca2+ Mn2+, Co2+ Ni2+, Zn2+, Pb2+, Cd2+, Hg2+, Ag+, Hg2+ and Al3+ in 1:1 v/v CH3CN:H2O. These metal ions do not interfere the fluorescent/colorimetric sensing. As fluorescent sensor the linear range of detection is 5 × 10−5 to 3 × 10−4 M and detection limit 10−5 M.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 138, 5 March 2015, Pages 99–104