| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230142 | 1495218 | 2015 | 6 صفحه PDF | دانلود رایگان |
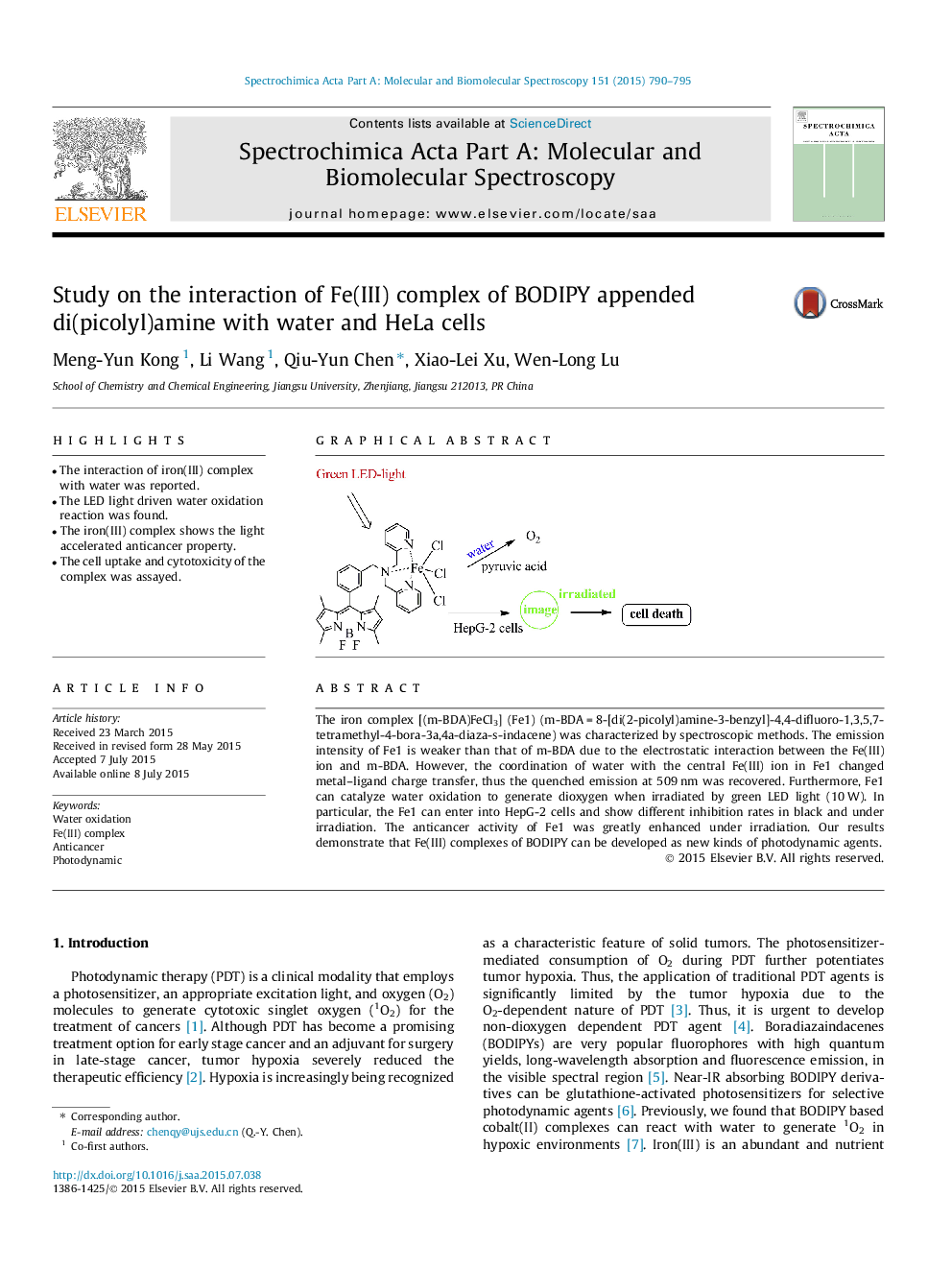
• The interaction of iron(III) complex with water was reported.
• The LED light driven water oxidation reaction was found.
• The iron(III) complex shows the light accelerated anticancer property.
• The cell uptake and cytotoxicity of the complex was assayed.
The iron complex [(m-BDA)FeCl3] (Fe1) (m-BDA = 8-[di(2-picolyl)amine-3-benzyl]-4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene) was characterized by spectroscopic methods. The emission intensity of Fe1 is weaker than that of m-BDA due to the electrostatic interaction between the Fe(III) ion and m-BDA. However, the coordination of water with the central Fe(III) ion in Fe1 changed metal–ligand charge transfer, thus the quenched emission at 509 nm was recovered. Furthermore, Fe1 can catalyze water oxidation to generate dioxygen when irradiated by green LED light (10 W). In particular, the Fe1 can enter into HepG-2 cells and show different inhibition rates in black and under irradiation. The anticancer activity of Fe1 was greatly enhanced under irradiation. Our results demonstrate that Fe(III) complexes of BODIPY can be developed as new kinds of photodynamic agents.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 151, 5 December 2015, Pages 790–795