| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230300 | 1495246 | 2014 | 8 صفحه PDF | دانلود رایگان |
• Cu(II)-NSAIDs show strong binding affinity to polydG–dC and polydG–polydC.
• Their binding is sensitive to differences in structure and hydration pattern of GC bases.
• They respond to it by showing different binding parameters and binding modes.
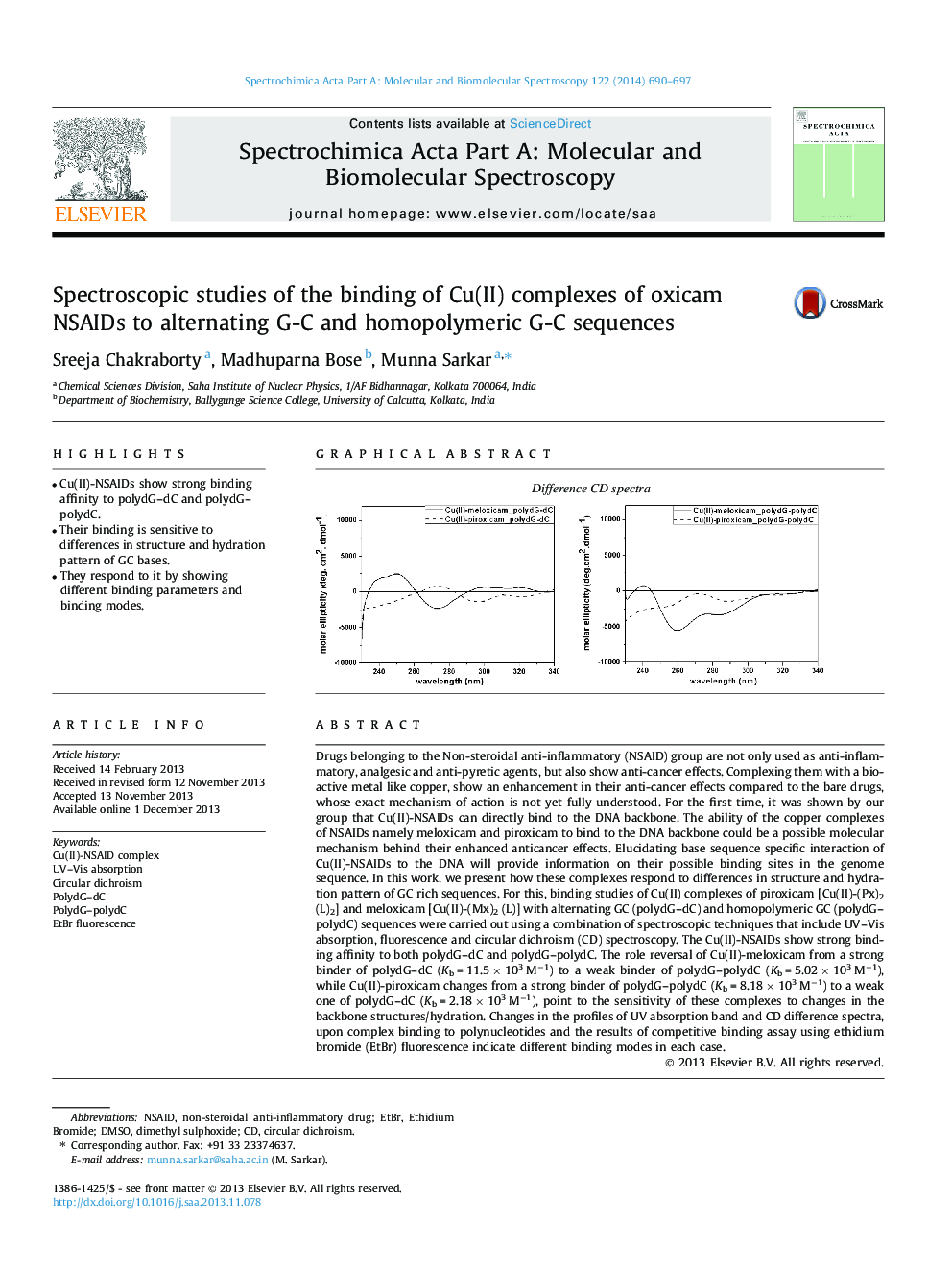
Drugs belonging to the Non-steroidal anti-inflammatory (NSAID) group are not only used as anti-inflammatory, analgesic and anti-pyretic agents, but also show anti-cancer effects. Complexing them with a bioactive metal like copper, show an enhancement in their anti-cancer effects compared to the bare drugs, whose exact mechanism of action is not yet fully understood. For the first time, it was shown by our group that Cu(II)-NSAIDs can directly bind to the DNA backbone. The ability of the copper complexes of NSAIDs namely meloxicam and piroxicam to bind to the DNA backbone could be a possible molecular mechanism behind their enhanced anticancer effects. Elucidating base sequence specific interaction of Cu(II)-NSAIDs to the DNA will provide information on their possible binding sites in the genome sequence. In this work, we present how these complexes respond to differences in structure and hydration pattern of GC rich sequences. For this, binding studies of Cu(II) complexes of piroxicam [Cu(II)-(Px)2 (L)2] and meloxicam [Cu(II)-(Mx)2 (L)] with alternating GC (polydG–dC) and homopolymeric GC (polydG–polydC) sequences were carried out using a combination of spectroscopic techniques that include UV–Vis absorption, fluorescence and circular dichroism (CD) spectroscopy. The Cu(II)-NSAIDs show strong binding affinity to both polydG–dC and polydG–polydC. The role reversal of Cu(II)-meloxicam from a strong binder of polydG–dC (Kb = 11.5 × 103 M−1) to a weak binder of polydG–polydC (Kb = 5.02 × 103 M−1), while Cu(II)-piroxicam changes from a strong binder of polydG–polydC (Kb = 8.18 × 103 M−1) to a weak one of polydG–dC (Kb = 2.18 × 103 M−1), point to the sensitivity of these complexes to changes in the backbone structures/hydration. Changes in the profiles of UV absorption band and CD difference spectra, upon complex binding to polynucleotides and the results of competitive binding assay using ethidium bromide (EtBr) fluorescence indicate different binding modes in each case.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 122, 25 March 2014, Pages 690–697