| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230692 | 1495261 | 2013 | 8 صفحه PDF | دانلود رایگان |
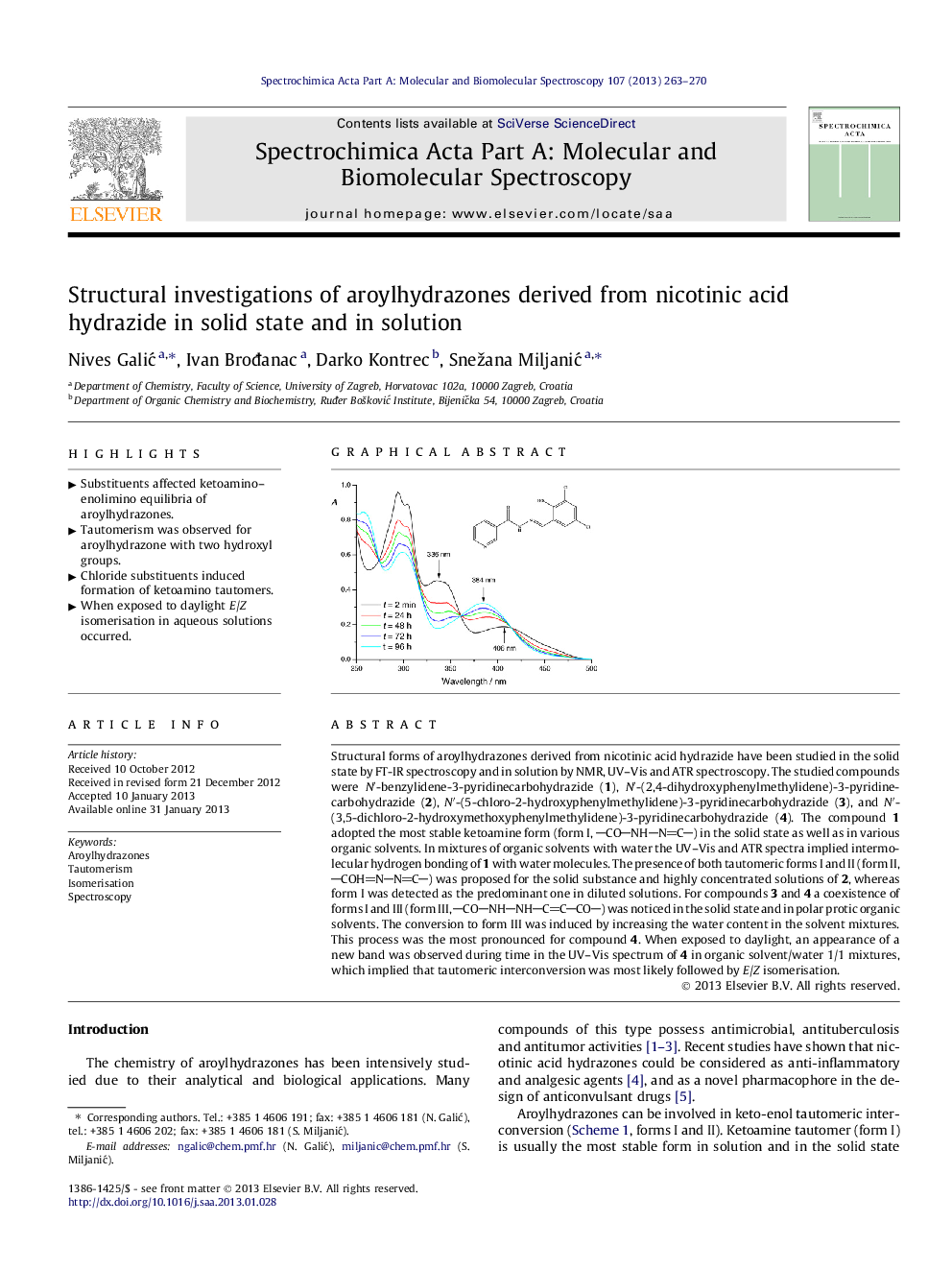
Structural forms of aroylhydrazones derived from nicotinic acid hydrazide have been studied in the solid state by FT-IR spectroscopy and in solution by NMR, UV–Vis and ATR spectroscopy. The studied compounds were N′-benzylidene-3-pyridinecarbohydrazide (1), N′-(2,4-dihydroxyphenylmethylidene)-3-pyridinecarbohydrazide (2), N′-(5-chloro-2-hydroxyphenylmethylidene)-3-pyridinecarbohydrazide (3), and N′-(3,5-dichloro-2-hydroxymethoxyphenylmethylidene)-3-pyridinecarbohydrazide (4). The compound 1 adopted the most stable ketoamine form (form I, CONHNC) in the solid state as well as in various organic solvents. In mixtures of organic solvents with water the UV–Vis and ATR spectra implied intermolecular hydrogen bonding of 1 with water molecules. The presence of both tautomeric forms I and II (form II, COHNNC) was proposed for the solid substance and highly concentrated solutions of 2, whereas form I was detected as the predominant one in diluted solutions. For compounds 3 and 4 a coexistence of forms I and III (form III, CONHNHCCCO) was noticed in the solid state and in polar protic organic solvents. The conversion to form III was induced by increasing the water content in the solvent mixtures. This process was the most pronounced for compound 4. When exposed to daylight, an appearance of a new band was observed during time in the UV–Vis spectrum of 4 in organic solvent/water 1/1 mixtures, which implied that tautomeric interconversion was most likely followed by E/Z isomerisation.
Figure optionsDownload as PowerPoint slideHighlights
► Substituents affected ketoamino–enolimino equilibria of aroylhydrazones.
► Tautomerism was observed for aroylhydrazone with two hydroxyl groups.
► Chloride substituents induced formation of ketoamino tautomers.
► When exposed to daylight E/Z isomerisation in aqueous solutions occurred.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 107, 15 April 2013, Pages 263–270