| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230718 | 1495256 | 2013 | 16 صفحه PDF | دانلود رایگان |
• FT-IR and FT-Raman spectra of Phenyl-N-(4-Methyl Phenyl) Nitrone (PN4MPN) in the solid phase were recorded and analyzed.
• Vibrational assignments PED of PN4MPN were calculated.
• The first order hyperpolarizability and HOMO, LUMO energy gap were theoretically predicted.
• The electron density-based local reactivity descriptor such as Fukui functions is calculated.
The title compound, Phenyl-N-(4-Methyl Phenyl) Nitrone (PN4MPN) was synthesized and characterized by FT-IR, FT-Raman and 1HNMR, 13CNMR spectral analysis. The molecular geometry, harmonic vibrational frequencies and bonding features of the title compound in the ground state are computed at the Hartree–Fock/6-311++G(d,p) and three parameter hybrid functional Lee–Yang–Parr/6-311++G(d,p) levels of theory. The calculated results show that the predicted geometry can well reproduce the structural parameters. The assignments of the vibrational spectra have been carried out with the help of normal co-ordinate analysis (NCA) following the scaled quantum mechanical force field methodology (SQMF). The calculated HOMO and LUMO energies confirm that charge transfer occurs within the molecule. The dipole moment (μ), polarizability (α) and hyperpolarizability (β) of the investigated molecule is calculated by using HF/6-311++G(d,p) and B3LYP/6-311++G(d,p) methods on the finite field approach. Besides, Molecular Electrostatic Potential (MEP), Natural Bond Orbital analysis (NBO) and thermodynamical properties are described from the computational process. The electron density-based local reactivity descriptor such as Fukui functions are calculated to explain the chemical selectivity or reactivity site in PN4MPN. Finally, the calculations are applied to simulated FT-IR and FT-Raman spectra of the title compound which show good agreement with observed spectra.
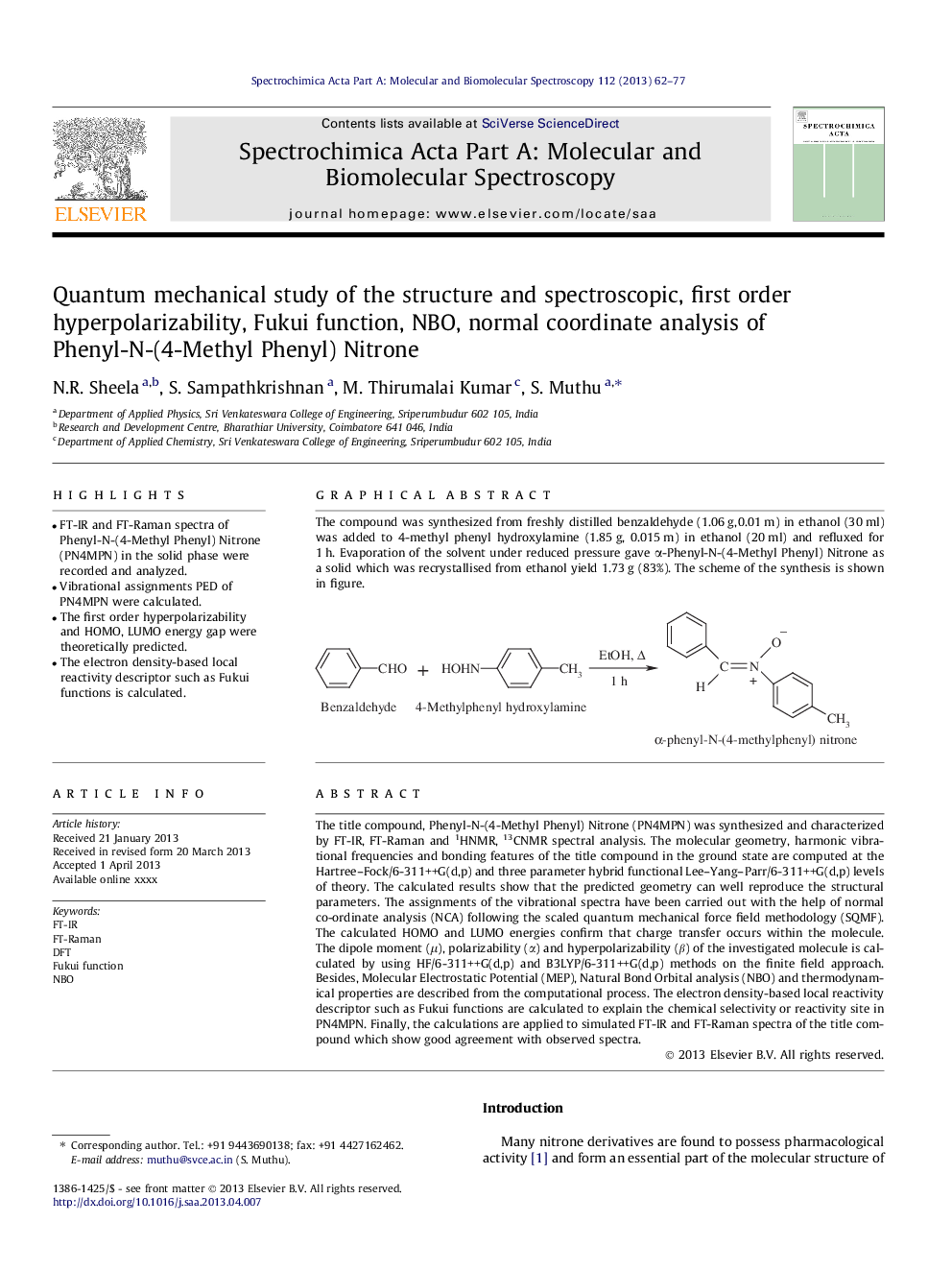
The compound was synthesized from freshly distilled benzaldehyde (1.06 g, 0.01 m) in ethanol (30 ml) was added to 4-methyl phenyl hydroxylamine (1.85 g, 0.015 m) in ethanol (20 ml) and refluxed for 1 h. Evaporation of the solvent under reduced pressure gave α-Phenyl-N-(4-Methyl Phenyl) Nitrone as a solid which was recrystallised from ethanol yield 1.73 g (83%). The scheme of the synthesis is shown in figure.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 112, August 2013, Pages 62–77