| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230745 | 1495256 | 2013 | 4 صفحه PDF | دانلود رایگان |
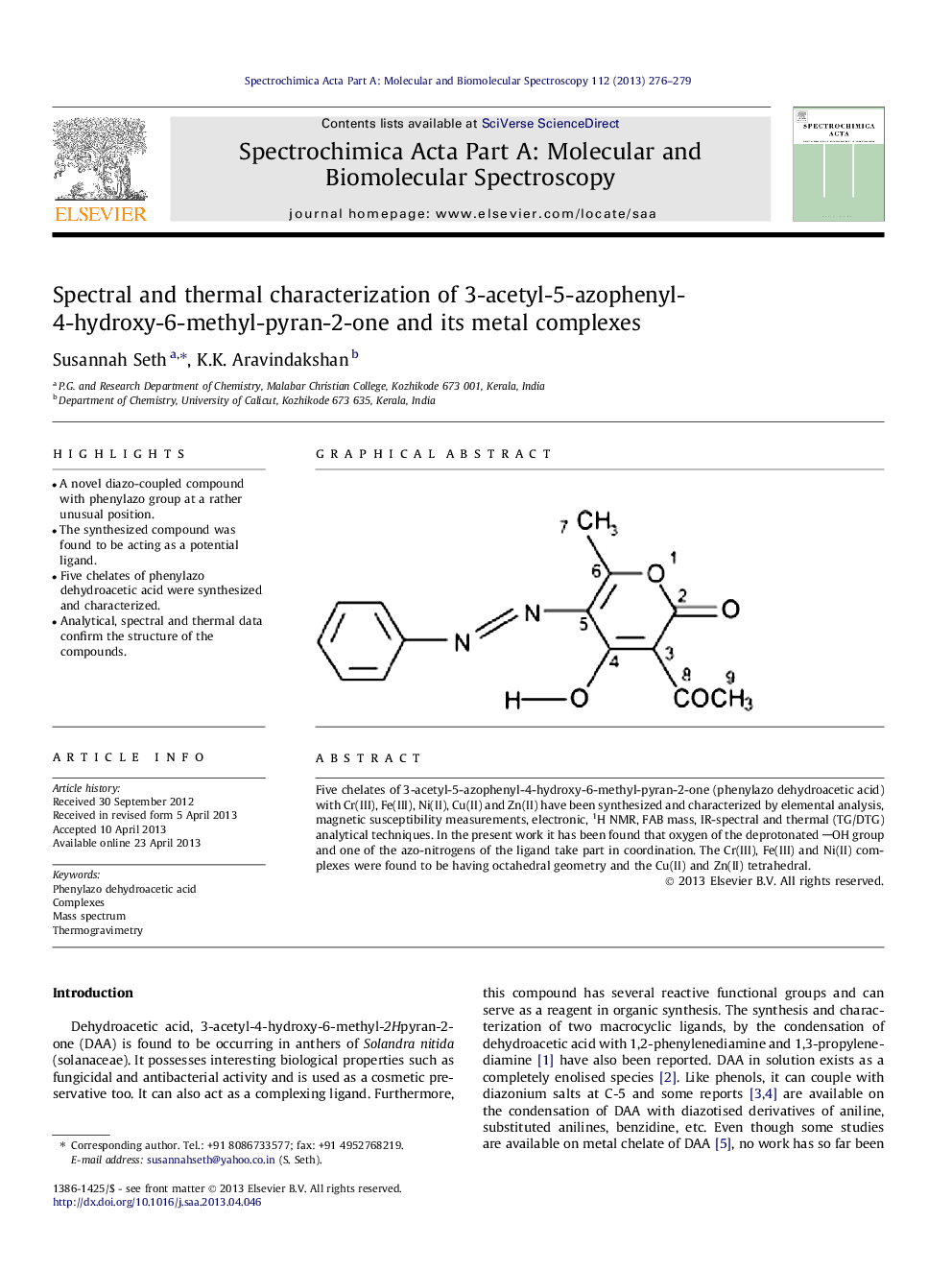
• A novel diazo-coupled compound with phenylazo group at a rather unusual position.
• The synthesized compound was found to be acting as a potential ligand.
• Five chelates of phenylazo dehydroacetic acid were synthesized and characterized.
• Analytical, spectral and thermal data confirm the structure of the compounds.
Five chelates of 3-acetyl-5-azophenyl-4-hydroxy-6-methyl-pyran-2-one (phenylazo dehydroacetic acid) with Cr(III), Fe(III), Ni(II), Cu(II) and Zn(II) have been synthesized and characterized by elemental analysis, magnetic susceptibility measurements, electronic, 1H NMR, FAB mass, IR-spectral and thermal (TG/DTG) analytical techniques. In the present work it has been found that oxygen of the deprotonated OH group and one of the azo-nitrogens of the ligand take part in coordination. The Cr(III), Fe(III) and Ni(II) complexes were found to be having octahedral geometry and the Cu(II) and Zn(II) tetrahedral.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 112, August 2013, Pages 276–279