| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230846 | 1495248 | 2014 | 15 صفحه PDF | دانلود رایگان |
• Vibrational spectra of 4-HTMDBN is recorded and analyzed.
• Vibrational assignments PED of 4-HTMDBN were calculated.
• The electron density and Fukui functions are calculated.
• The NLO and thermodynamic properties are calculated theoretically.
The Fourier transform infrared (FTIR) and FT Raman (FTR) of 4-4′-(1H-1, 2, 4-triazol-1-yl methylene) dibenzonitrile (4-HTMDBN) have been recorded and analyzed. The equilibrium geometry harmonic vibrational frequencies have been investigated with the help of standard HF and DFT methods with 6-31G(d,p) as basis set. The assignments of the vibrational spectra have been carried out with the help of normal co-ordinate analysis (NCA) following the scaled quantum mechanical force field methodology (SQMFF). Theoretical simulations of the FTIR and FTR spectra of the title compound have been calculated. The 1H and 13C Nuclear Magnetic Resonance (NMR) chemical shifts of the molecule were calculated by the Gauge including atomic orbital (GIAO) method. The stability of the molecule has been analyzed using natural bond orbital (NBO) analysis. The linear polarizability (α) and the first order hyperpolarizability (β) values of the investigated molecule have been computed using HF/DFT/6-31G(d,p) methods on the finite field approach. UV–Vis spectrum of the compound is recorded and the electronic properties such as HOMO and LUMO energies, are performed. The directly calculated ionization potential (IP), electron affinity (EA), electronegativity (χ), electrophilicity index (ω), hardness (η) and chemical potential (ρ) are all correlated with the HOMO and LUMO energies with their molecular properties. Mulliken population analysis on atomic charges, molecular electrostatic potential maps (MEP) and thermodynamical properties of title compound at different temperature have been calculated.
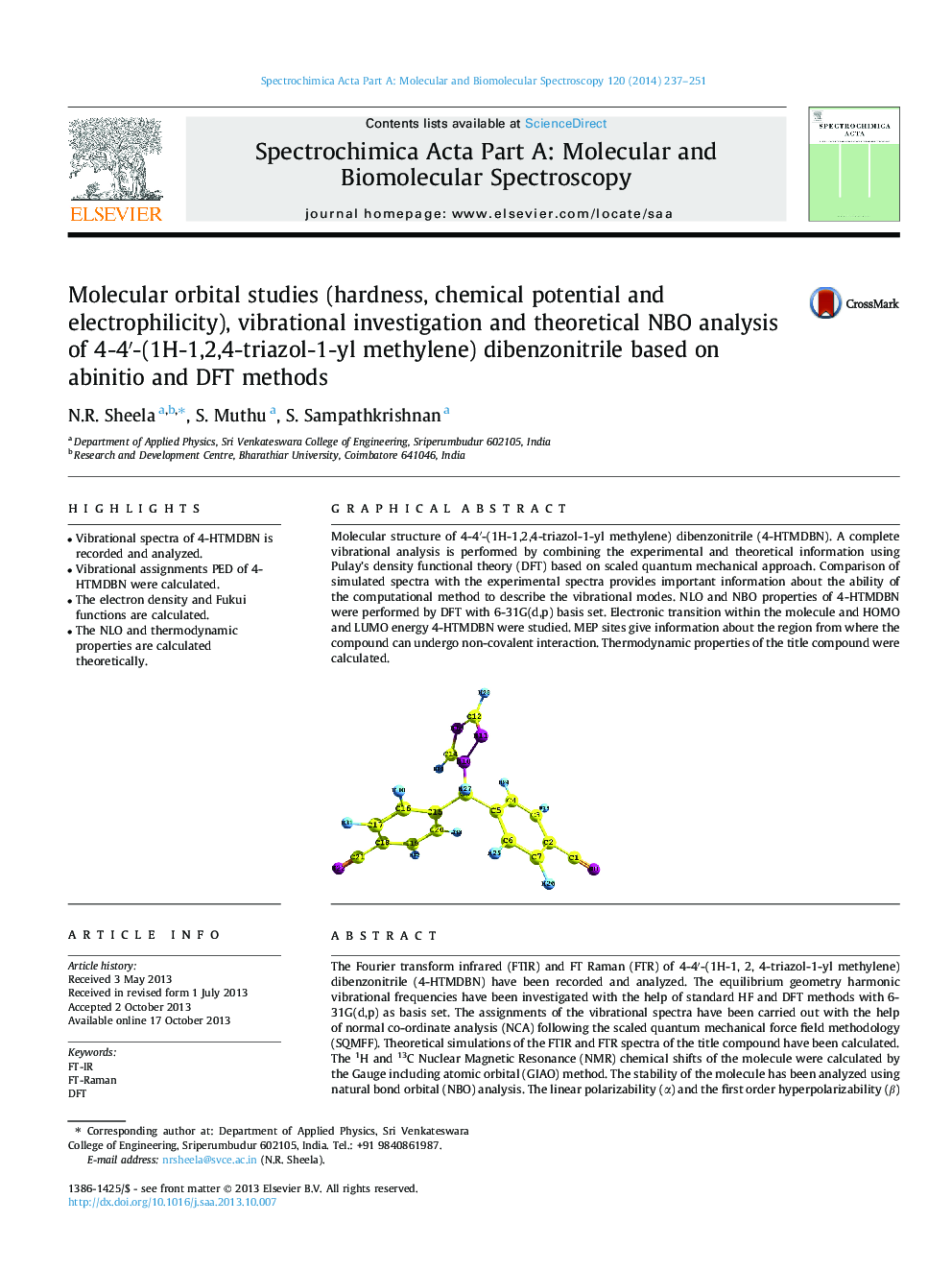
Molecular structure of 4-4′-(1H-1,2,4-triazol-1-yl methylene) dibenzonitrile (4-HTMDBN). A complete vibrational analysis is performed by combining the experimental and theoretical information using Pulay’s density functional theory (DFT) based on scaled quantum mechanical approach. Comparison of simulated spectra with the experimental spectra provides important information about the ability of the computational method to describe the vibrational modes. NLO and NBO properties of 4-HTMDBN were performed by DFT with 6-31G(d,p) basis set. Electronic transition within the molecule and HOMO and LUMO energy 4-HTMDBN were studied. MEP sites give information about the region from where the compound can undergo non-covalent interaction. Thermodynamic properties of the title compound were calculated.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 120, 24 February 2014, Pages 237–251