| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231522 | 1495259 | 2013 | 13 صفحه PDF | دانلود رایگان |
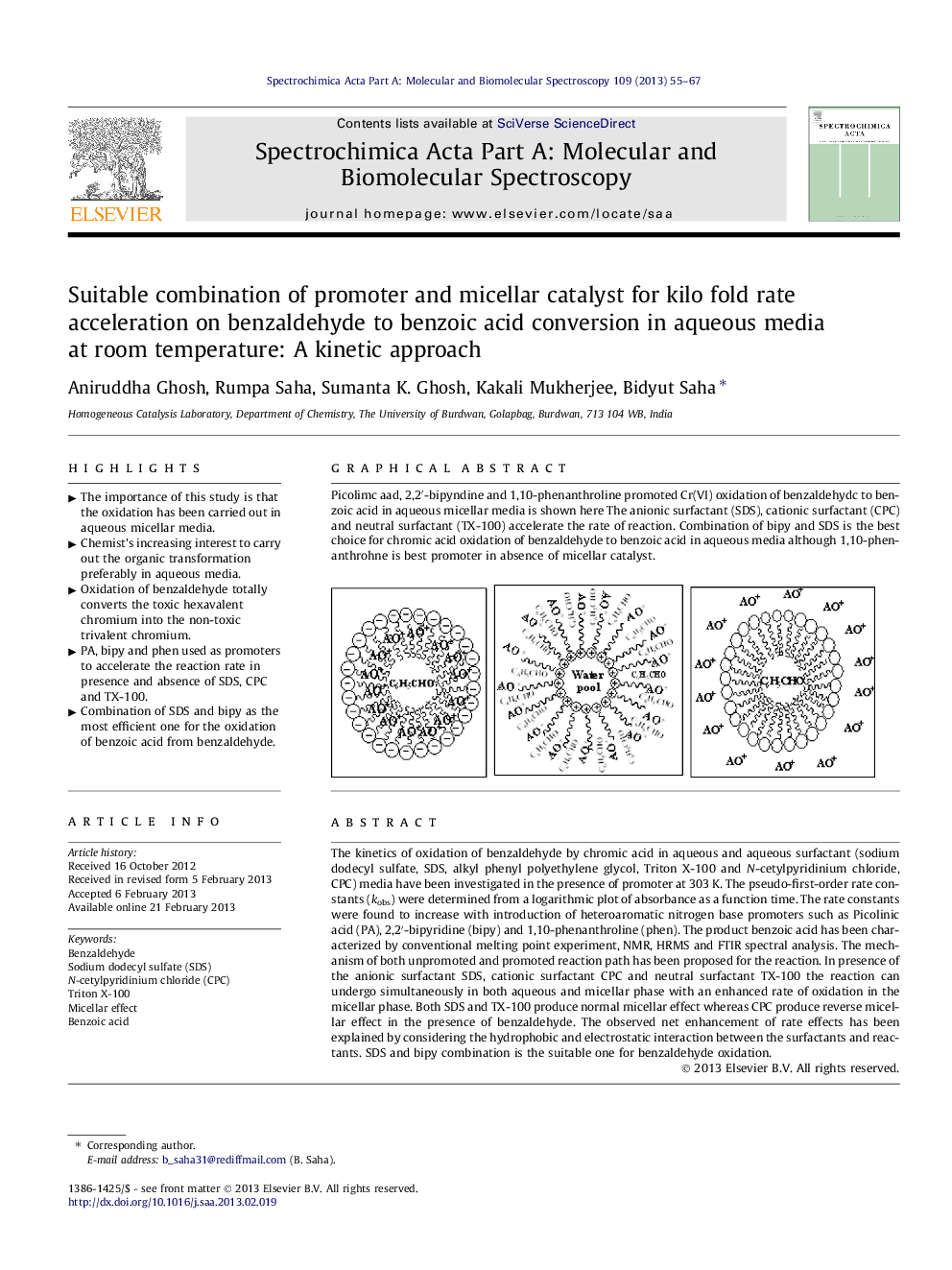
The kinetics of oxidation of benzaldehyde by chromic acid in aqueous and aqueous surfactant (sodium dodecyl sulfate, SDS, alkyl phenyl polyethylene glycol, Triton X-100 and N-cetylpyridinium chloride, CPC) media have been investigated in the presence of promoter at 303 K. The pseudo-first-order rate constants (kobs) were determined from a logarithmic plot of absorbance as a function time. The rate constants were found to increase with introduction of heteroaromatic nitrogen base promoters such as Picolinic acid (PA), 2,2′-bipyridine (bipy) and 1,10-phenanthroline (phen). The product benzoic acid has been characterized by conventional melting point experiment, NMR, HRMS and FTIR spectral analysis. The mechanism of both unpromoted and promoted reaction path has been proposed for the reaction. In presence of the anionic surfactant SDS, cationic surfactant CPC and neutral surfactant TX-100 the reaction can undergo simultaneously in both aqueous and micellar phase with an enhanced rate of oxidation in the micellar phase. Both SDS and TX-100 produce normal micellar effect whereas CPC produce reverse micellar effect in the presence of benzaldehyde. The observed net enhancement of rate effects has been explained by considering the hydrophobic and electrostatic interaction between the surfactants and reactants. SDS and bipy combination is the suitable one for benzaldehyde oxidation.
Picolimc aad, 2,2′-bipyndine and 1,10-phenanthroline promoted Cr(VI) oxidation of benzaldehydc to benzoic acid in aqueous micellar media is shown here The anionic surfactant (SDS), cationic surfactant (CPC) and neutral surfactant (TX-100) accelerate the rate of reaction. Combination of bipy and SDS is the best choice for chromic acid oxidation of benzaldehyde to benzoic acid in aqueous media although 1,10-phenanthrohne is best promoter in absence of micellar catalyst.Figure optionsDownload as PowerPoint slideHighlights
► The importance of this study is that the oxidation has been carried out in aqueous micellar media.
► Chemist’s increasing interest to carry out the organic transformation preferably in aqueous media.
► Oxidation of benzaldehyde totally converts the toxic hexavalent chromium into the non-toxic trivalent chromium.
► PA, bipy and phen used as promoters to accelerate the reaction rate in presence and absence of SDS, CPC and TX-100.
► Combination of SDS and bipy as the most efficient one for the oxidation of benzoic acid from benzaldehyde.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 109, 15 May 2013, Pages 55–67