| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232291 | 1495271 | 2012 | 6 صفحه PDF | دانلود رایگان |
To know the interaction of amphiphilic drugs nortriptyline hydrochloride (NOT) and promazine hydrochloride (PMZ) with serum albumins (i.e., human serum albumin (HSA) and bovine serum albumin (BSA)), techniques of UV–visible, fluorescence, and circular dichroism (CD) spectroscopies are used. The binding affinity is more in case of PMZ with both the serum albumins. The quenching rate constant (kq) values suggest a static quenching process for all the drug–serum albumin interactions. The UV–visible results show that the change in protein conformation of PMZ–serum albumin interactions are more prominent as compared to NOT–serum albumin interactions. The CD results also explain the conformational changes in the serum albumins on binding with the drugs. The increment in %α-helical structure is slightly more for drug–BSA complexes as compared to drug–HSA complexes.
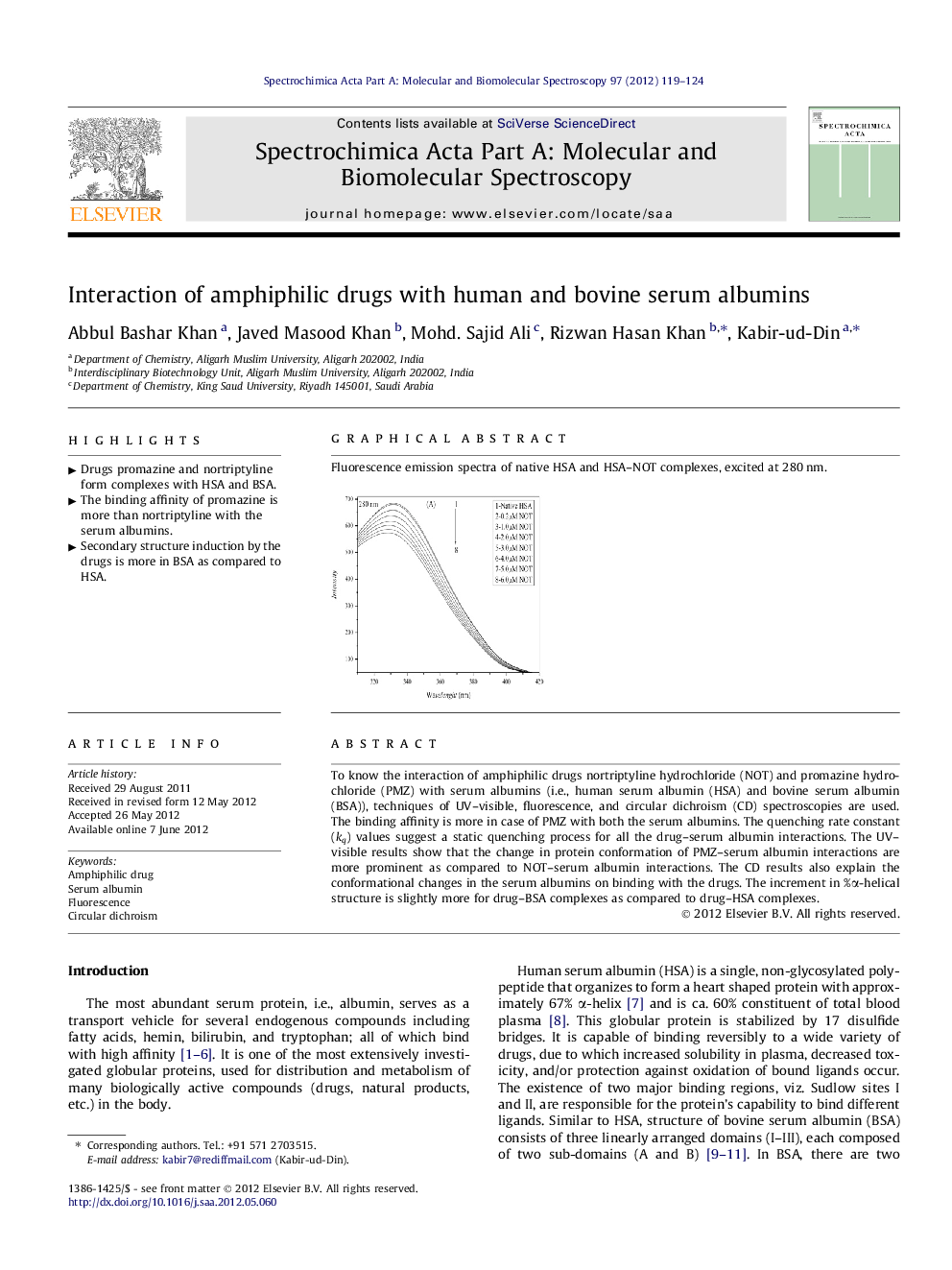
Fluorescence emission spectra of native HSA and HSA–NOT complexes, excited at 280 nm.Figure optionsDownload as PowerPoint slideHighlights
► Drugs promazine and nortriptyline form complexes with HSA and BSA.
► The binding affinity of promazine is more than nortriptyline with the serum albumins.
► Secondary structure induction by the drugs is more in BSA as compared to HSA.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 97, November 2012, Pages 119–124