| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232330 | 1495271 | 2012 | 6 صفحه PDF | دانلود رایگان |
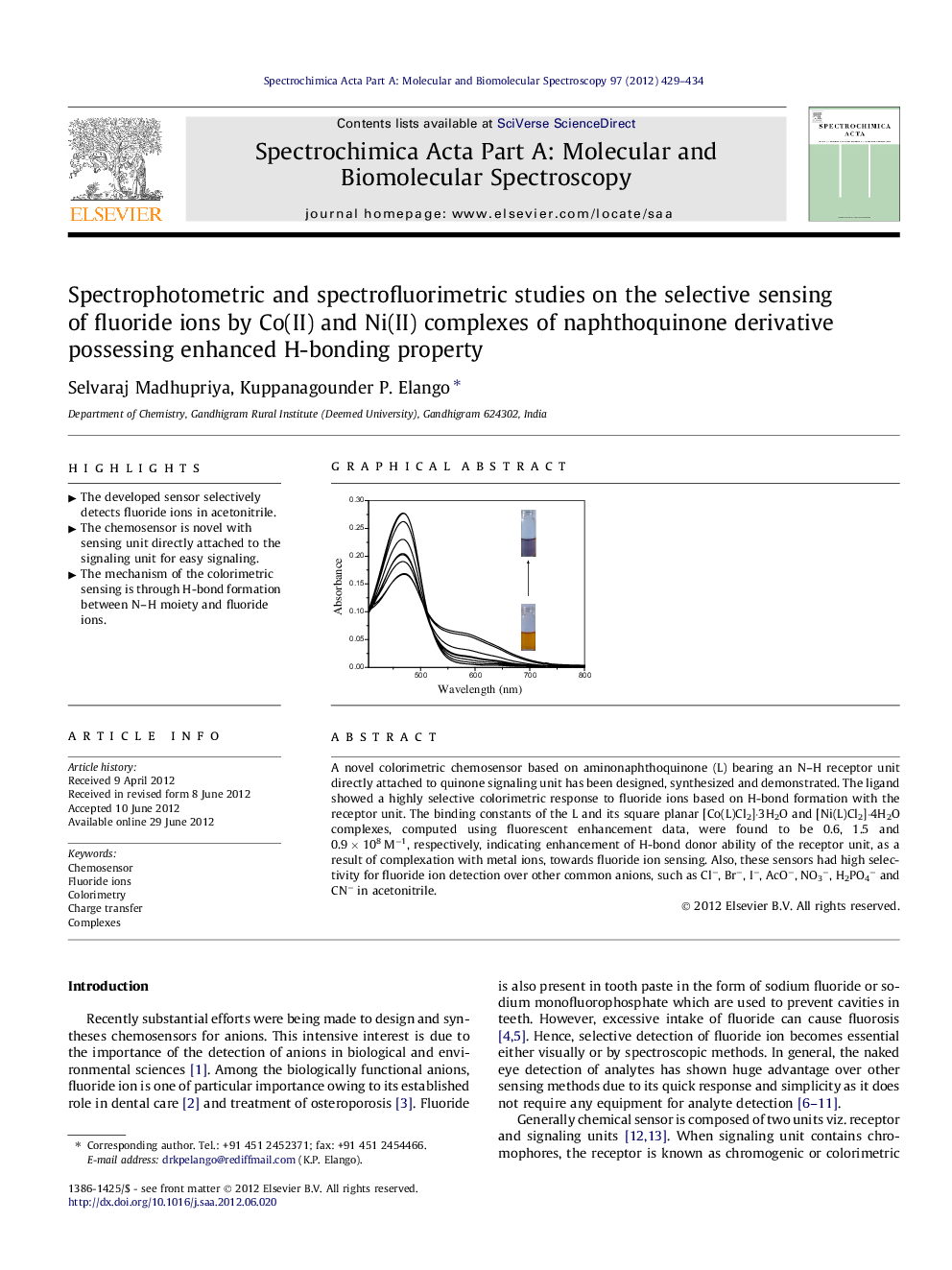
A novel colorimetric chemosensor based on aminonaphthoquinone (L) bearing an N–H receptor unit directly attached to quinone signaling unit has been designed, synthesized and demonstrated. The ligand showed a highly selective colorimetric response to fluoride ions based on H-bond formation with the receptor unit. The binding constants of the L and its square planar [Co(L)Cl2]·3H2O and [Ni(L)Cl2]·4H2O complexes, computed using fluorescent enhancement data, were found to be 0.6, 1.5 and 0.9 × 108 M−1, respectively, indicating enhancement of H-bond donor ability of the receptor unit, as a result of complexation with metal ions, towards fluoride ion sensing. Also, these sensors had high selectivity for fluoride ion detection over other common anions, such as Cl−, Br−, I−, AcO−, NO3−, H2PO4− and CN− in acetonitrile.
Figure optionsDownload as PowerPoint slideHighlights
► The developed sensor selectively detects fluoride ions in acetonitrile.
► The chemosensor is novel with sensing unit directly attached to the signaling unit for easy signaling.
► The mechanism of the colorimetric sensing is through H-bond formation between N–H moiety and fluoride ions.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 97, November 2012, Pages 429–434