| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232430 | 968792 | 2015 | 16 صفحه PDF | دانلود رایگان |
• The FT-IR, FT-Raman, spectral investigation of title molecule has been performed using DFT methods.
• Optimized parameters were calculated.
• The complete assignments are performed on the basis of the potential energy distribution (PED).
• The redistribution of electron density has been discussed.
• Hyperpolarizability, HOMO and LUMO energies were calculated.
In this work, the molecular structure, harmonic vibrational frequencies, UV, NBO and AIM of 3-thiophenecarboxilic acid (abbreviated as 3-TCA) monomer and dimer has been investigated. The FT-IR and FT-Raman spectra were recorded. The ground-state molecular geometry and vibrational frequencies have been calculated by using the Hartree–Fock (HF) and density functional theory (DFT)/B3LYP methods and 6-311++G(d,p) as a basis set. The fundamental vibrations were assigned on the basis of the total energy distribution (TED) of the vibrational modes, calculated with VEDA program. Comparison of the observed fundamental vibrational frequencies of 3-TCA with calculated results by HF and DFT methods indicates that B3LYP is better to HF method for molecular vibrational problems. The difference between the observed and scaled wavenumber values is very small. The theoretically predicted FT-IR and FT-Raman spectra of the title compound have been constructed. A study on the Mulliken atomic charges, the electronic properties were performed by time-dependent DFT (TD-DFT) approach, frontier molecular orbitals (HOMO–LUMO), molecular electrostatic potential (MEP) and thermodynamic properties have been performed. The electric dipole moment (μ) and the first hyperpolarizability (β) values of the investigated molecule have been also computed.
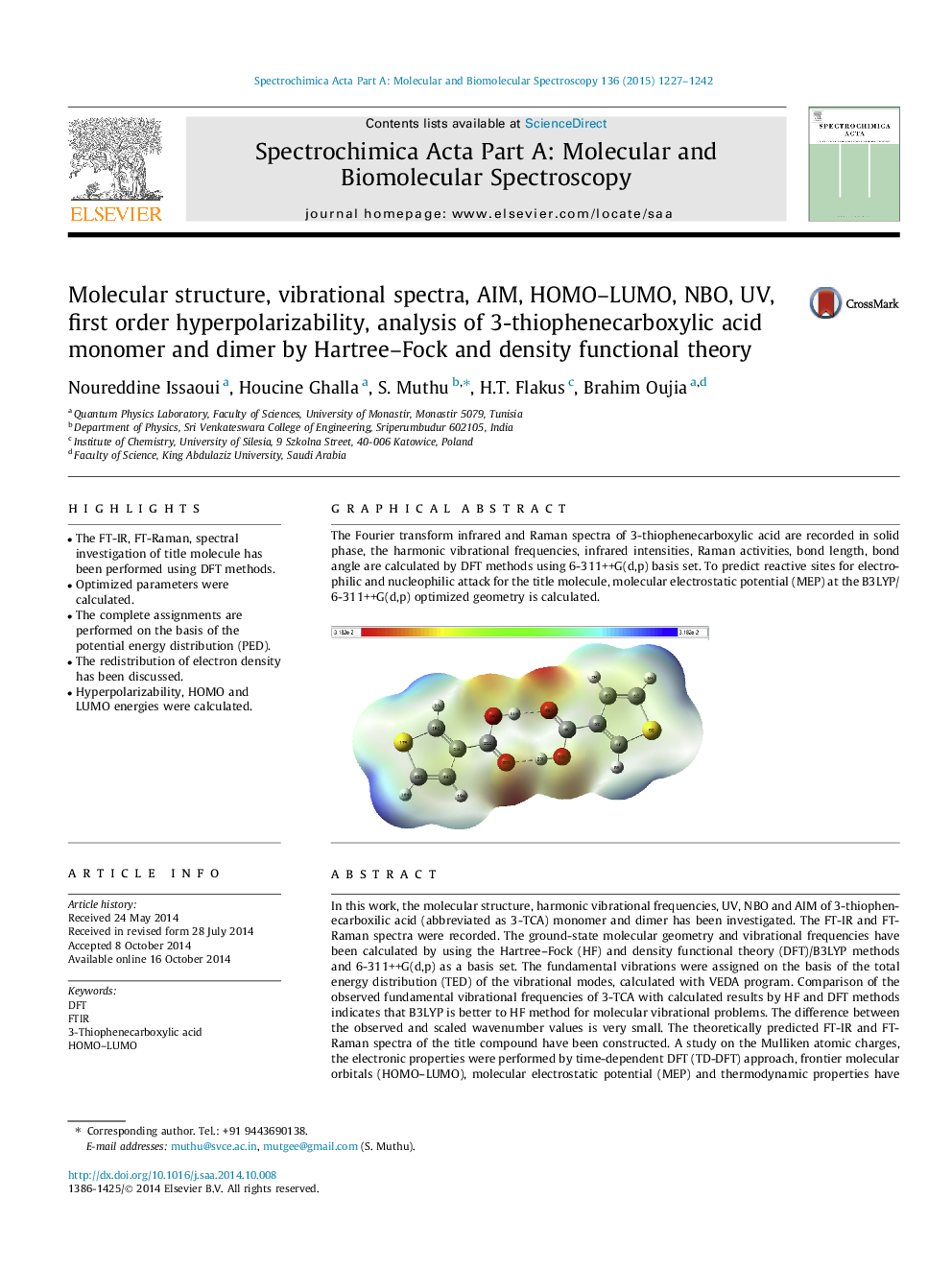
The Fourier transform infrared and Raman spectra of 3-thiophenecarboxylic acid are recorded in solid phase, the harmonic vibrational frequencies, infrared intensities, Raman activities, bond length, bond angle are calculated by DFT methods using 6-311++G(d,p) basis set. To predict reactive sites for electrophilic and nucleophilic attack for the title molecule, molecular electrostatic potential (MEP) at the B3LYP/6-311++G(d,p) optimized geometry is calculated.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part C, 5 February 2015, Pages 1227–1242