| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232462 | 968792 | 2015 | 15 صفحه PDF | دانلود رایگان |
• Optimized geometry and vibrational assignments were computed by DFT.
• NBO, NMR and UV spectral analysis were carried out.
• HOMO–LUMO and MEP analysis were made.
• Isotropic chemical shifts were calculated using the GIAO method.
• Local reactivity descriptor such as Fukui functions was calculated.
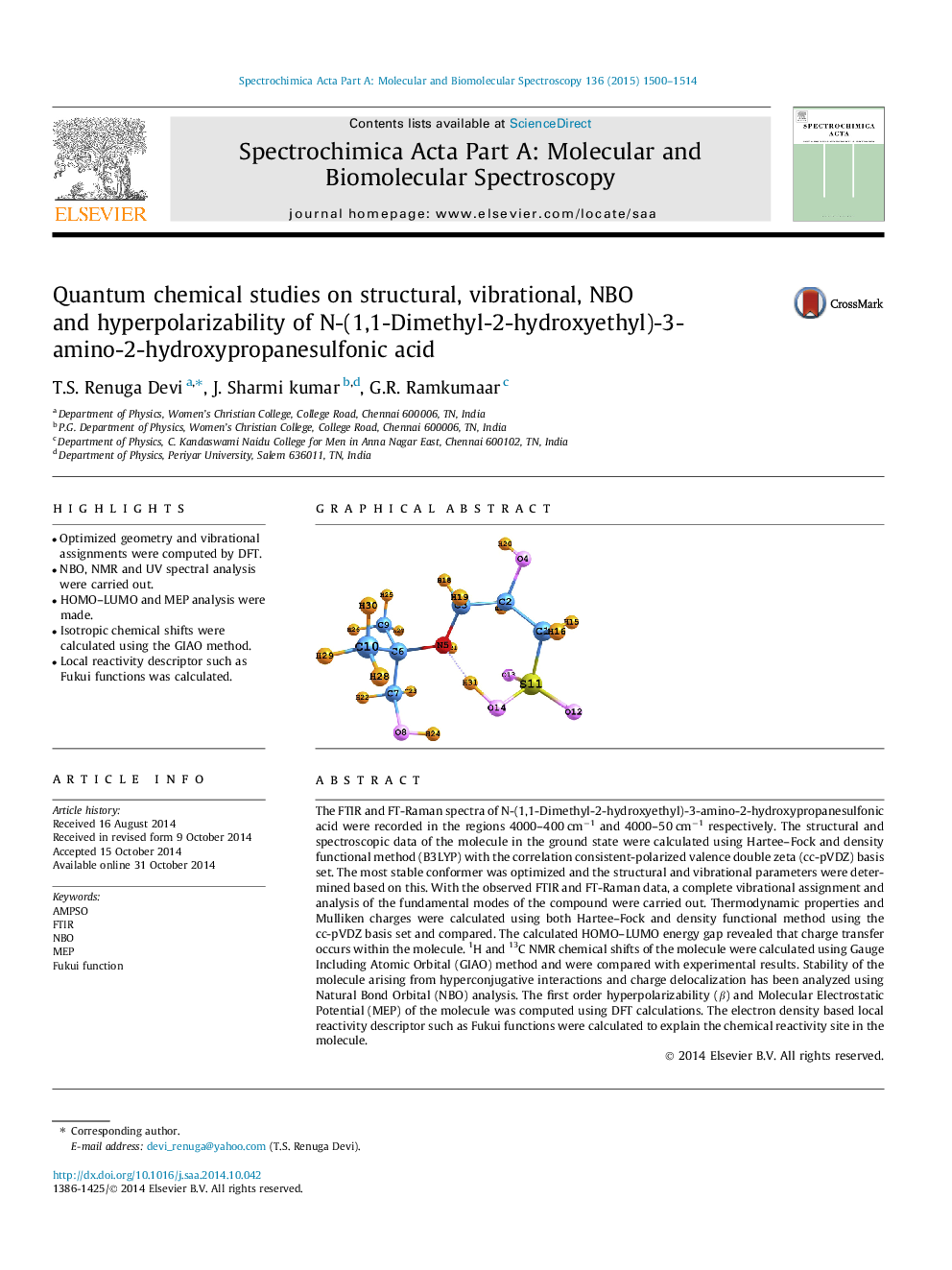
The FTIR and FT-Raman spectra of N-(1,1-Dimethyl-2-hydroxyethyl)-3-amino-2-hydroxypropanesulfonic acid were recorded in the regions 4000–400 cm−1 and 4000–50 cm−1 respectively. The structural and spectroscopic data of the molecule in the ground state were calculated using Hartee–Fock and density functional method (B3LYP) with the correlation consistent-polarized valence double zeta (cc-pVDZ) basis set. The most stable conformer was optimized and the structural and vibrational parameters were determined based on this. With the observed FTIR and FT-Raman data, a complete vibrational assignment and analysis of the fundamental modes of the compound were carried out. Thermodynamic properties and Mulliken charges were calculated using both Hartee–Fock and density functional method using the cc-pVDZ basis set and compared. The calculated HOMO–LUMO energy gap revealed that charge transfer occurs within the molecule. 1H and 13C NMR chemical shifts of the molecule were calculated using Gauge Including Atomic Orbital (GIAO) method and were compared with experimental results. Stability of the molecule arising from hyperconjugative interactions and charge delocalization has been analyzed using Natural Bond Orbital (NBO) analysis. The first order hyperpolarizability (β) and Molecular Electrostatic Potential (MEP) of the molecule was computed using DFT calculations. The electron density based local reactivity descriptor such as Fukui functions were calculated to explain the chemical reactivity site in the molecule.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part C, 5 February 2015, Pages 1500–1514