| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232502 | 968792 | 2015 | 8 صفحه PDF | دانلود رایگان |
• New transition metal complexes of 2,5-butanedione isonicotinylhydrazone.
• XRD structure of 2,5-butanedione isonicotinylhydrazone.
• The antimicrobial activity of the ligand and its complexes.
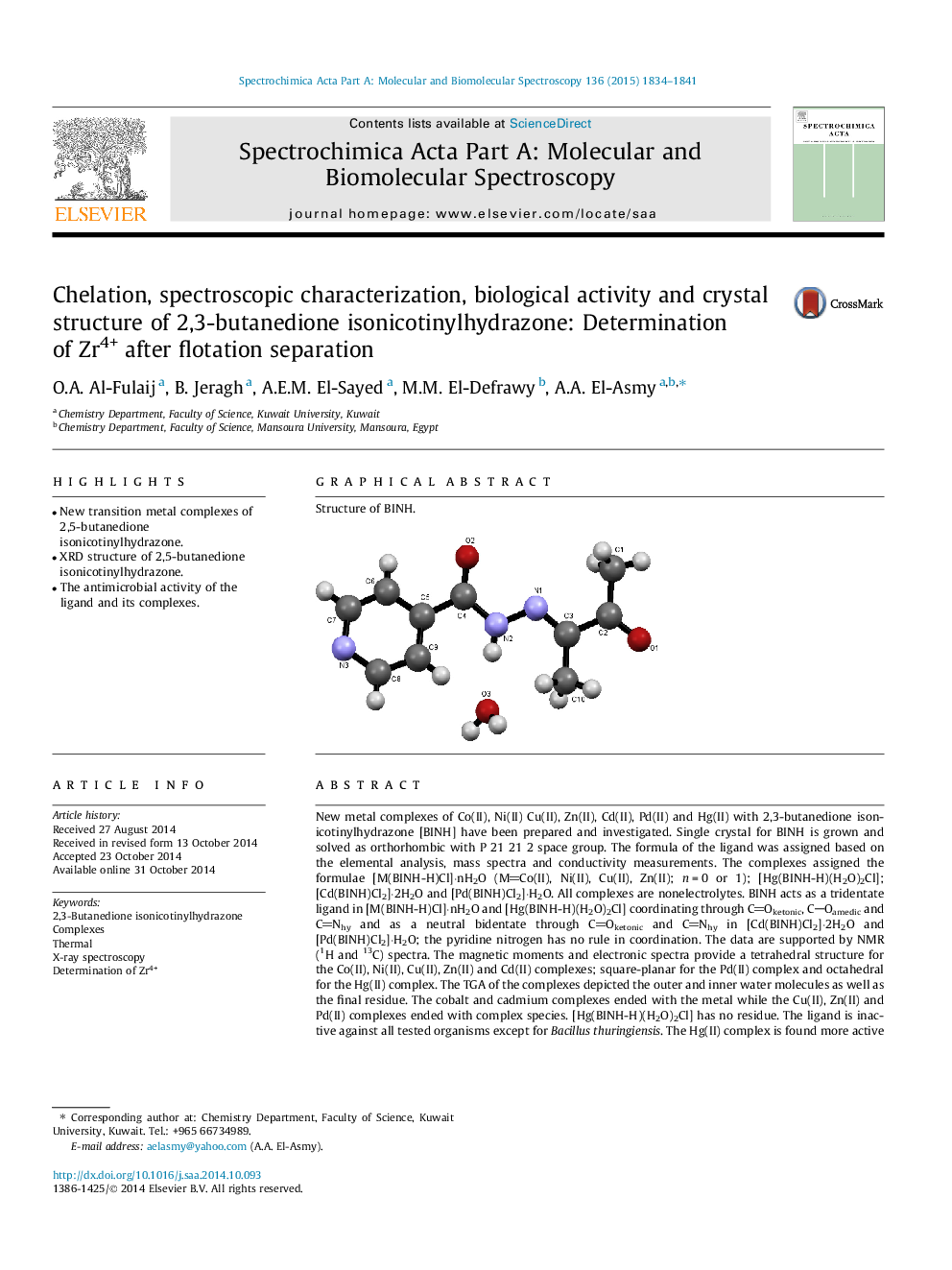
New metal complexes of Co(II), Ni(II) Cu(II), Zn(II), Cd(II), Pd(II) and Hg(II) with 2,3-butanedione isonicotinylhydrazone [BINH] have been prepared and investigated. Single crystal for BINH is grown and solved as orthorhombic with P 21 21 2 space group. The formula of the ligand was assigned based on the elemental analysis, mass spectra and conductivity measurements. The complexes assigned the formulae [M(BINH-H)Cl]⋅nH2O (MCo(II), Ni(II), Cu(II), Zn(II); n = 0 or 1); [Hg(BINH-H)(H2O)2Cl]; [Cd(BINH)Cl2]⋅2H2O and [Pd(BINH)Cl2]⋅H2O. All complexes are nonelectrolytes. BINH acts as a tridentate ligand in [M(BINH-H)Cl]⋅nH2O and [Hg(BINH-H)(H2O)2Cl] coordinating through COketonic, COamedic and CNhy and as a neutral bidentate through COketonic and CNhy in [Cd(BINH)Cl2]⋅2H2O and [Pd(BINH)Cl2]⋅H2O; the pyridine nitrogen has no rule in coordination. The data are supported by NMR (1H and 13C) spectra. The magnetic moments and electronic spectra provide a tetrahedral structure for the Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes; square-planar for the Pd(II) complex and octahedral for the Hg(II) complex. The TGA of the complexes depicted the outer and inner water molecules as well as the final residue. The cobalt and cadmium complexes ended with the metal while the Cu(II), Zn(II) and Pd(II) complexes ended with complex species. [Hg(BINH-H)(H2O)2Cl] has no residue. The ligand is inactive against all tested organisms except for Bacillus thuringiensis. The Hg(II) complex is found more active than the other complexes. The flotation technique is found applicable for the separation of micro amount (10 ppm) of Zr4+ using 10 ppm of BINH and 1 × 10−5 mol L−1 of oleic acid at pH 6 with efficiency of 98% with no interferences.
Structure of BINH.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part C, 5 February 2015, Pages 1834–1841