| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232843 | 1495245 | 2014 | 8 صفحه PDF | دانلود رایگان |
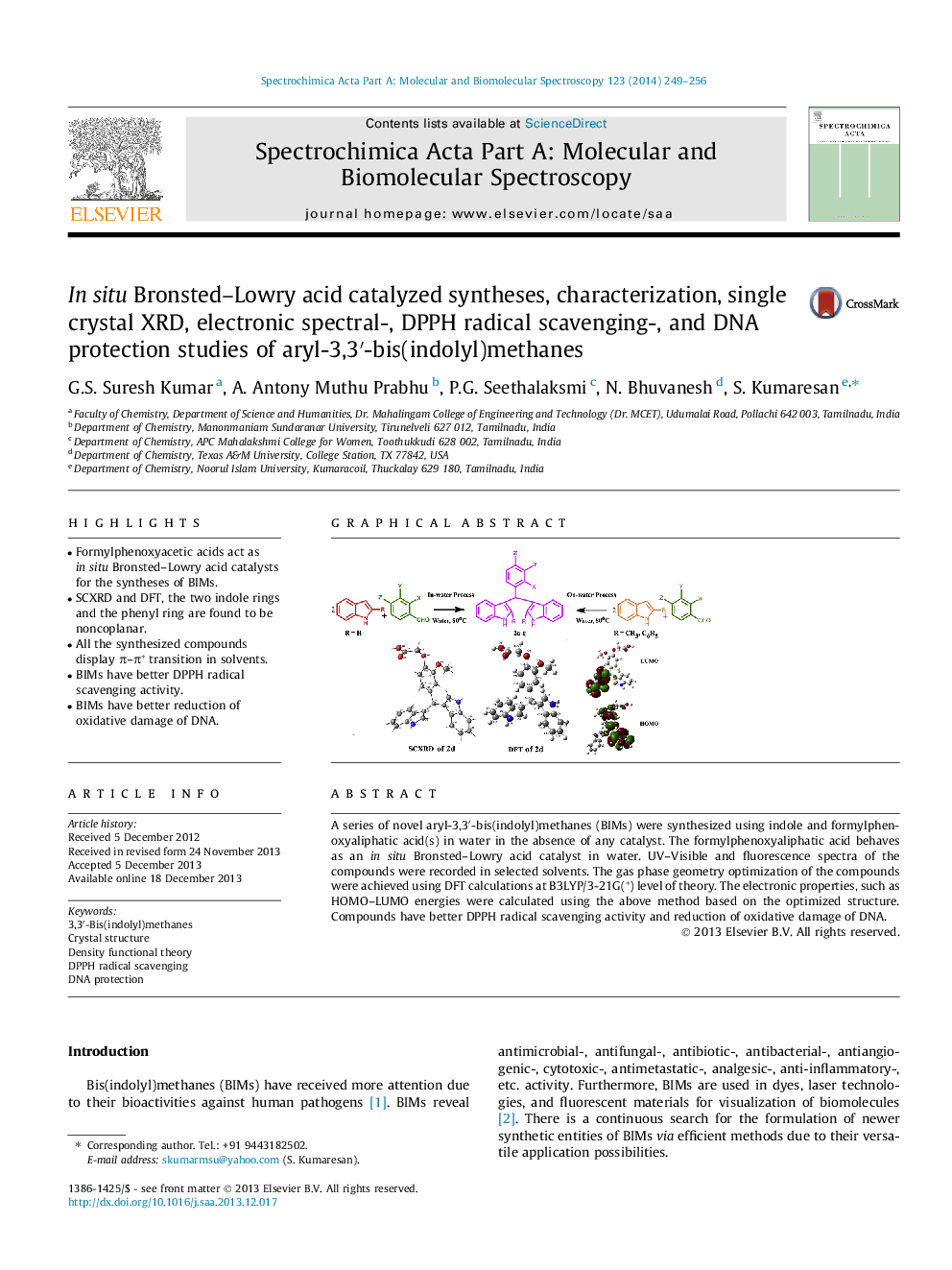
• Formylphenoxyacetic acids act as in situ Bronsted–Lowry acid catalysts for the syntheses of BIMs.
• SCXRD and DFT, the two indole rings and the phenyl ring are found to be noncoplanar.
• All the synthesized compounds display π–π* transition in solvents.
• BIMs have better DPPH radical scavenging activity.
• BIMs have better reduction of oxidative damage of DNA.
A series of novel aryl-3,3′-bis(indolyl)methanes (BIMs) were synthesized using indole and formylphenoxyaliphatic acid(s) in water in the absence of any catalyst. The formylphenoxyaliphatic acid behaves as an in situ Bronsted–Lowry acid catalyst in water. UV–Visible and fluorescence spectra of the compounds were recorded in selected solvents. The gas phase geometry optimization of the compounds were achieved using DFT calculations at B3LYP/3-21G(*) level of theory. The electronic properties, such as HOMO–LUMO energies were calculated using the above method based on the optimized structure. Compounds have better DPPH radical scavenging activity and reduction of oxidative damage of DNA.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 123, 5 April 2014, Pages 249–256