| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233426 | 1495235 | 2014 | 7 صفحه PDF | دانلود رایگان |
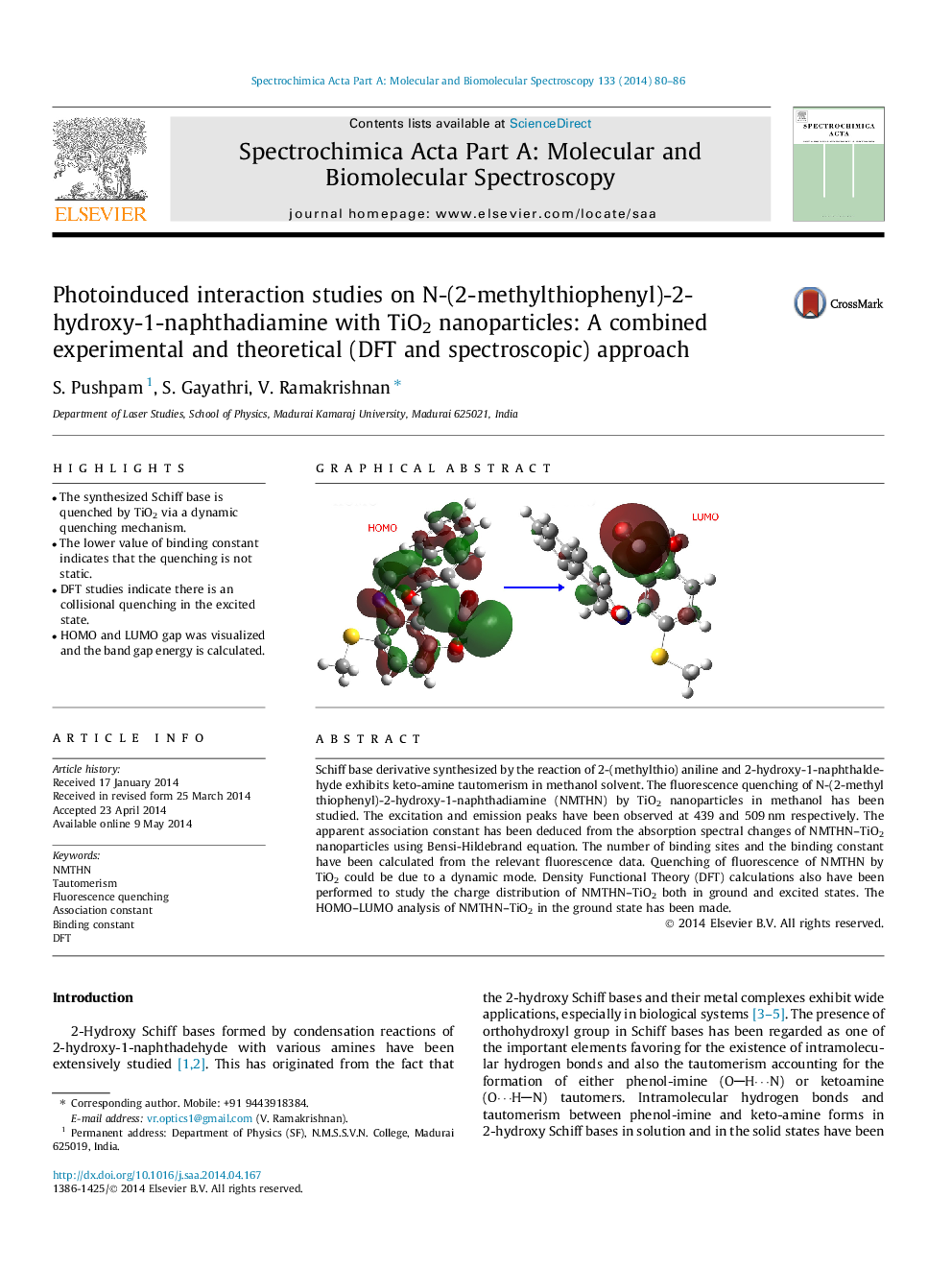
• The synthesized Schiff base is quenched by TiO2 via a dynamic quenching mechanism.
• The lower value of binding constant indicates that the quenching is not static.
• DFT studies indicate there is an collisional quenching in the excited state.
• HOMO and LUMO gap was visualized and the band gap energy is calculated.
Schiff base derivative synthesized by the reaction of 2-(methylthio) aniline and 2-hydroxy-1-naphthaldehyde exhibits keto-amine tautomerism in methanol solvent. The fluorescence quenching of N-(2-methyl thiophenyl)-2-hydroxy-1-naphthadiamine (NMTHN) by TiO2 nanoparticles in methanol has been studied. The excitation and emission peaks have been observed at 439 and 509 nm respectively. The apparent association constant has been deduced from the absorption spectral changes of NMTHN–TiO2 nanoparticles using Bensi-Hildebrand equation. The number of binding sites and the binding constant have been calculated from the relevant fluorescence data. Quenching of fluorescence of NMTHN by TiO2 could be due to a dynamic mode. Density Functional Theory (DFT) calculations also have been performed to study the charge distribution of NMTHN–TiO2 both in ground and excited states. The HOMO–LUMO analysis of NMTHN–TiO2 in the ground state has been made.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 133, 10 December 2014, Pages 80–86