| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233496 | 1495235 | 2014 | 6 صفحه PDF | دانلود رایگان |
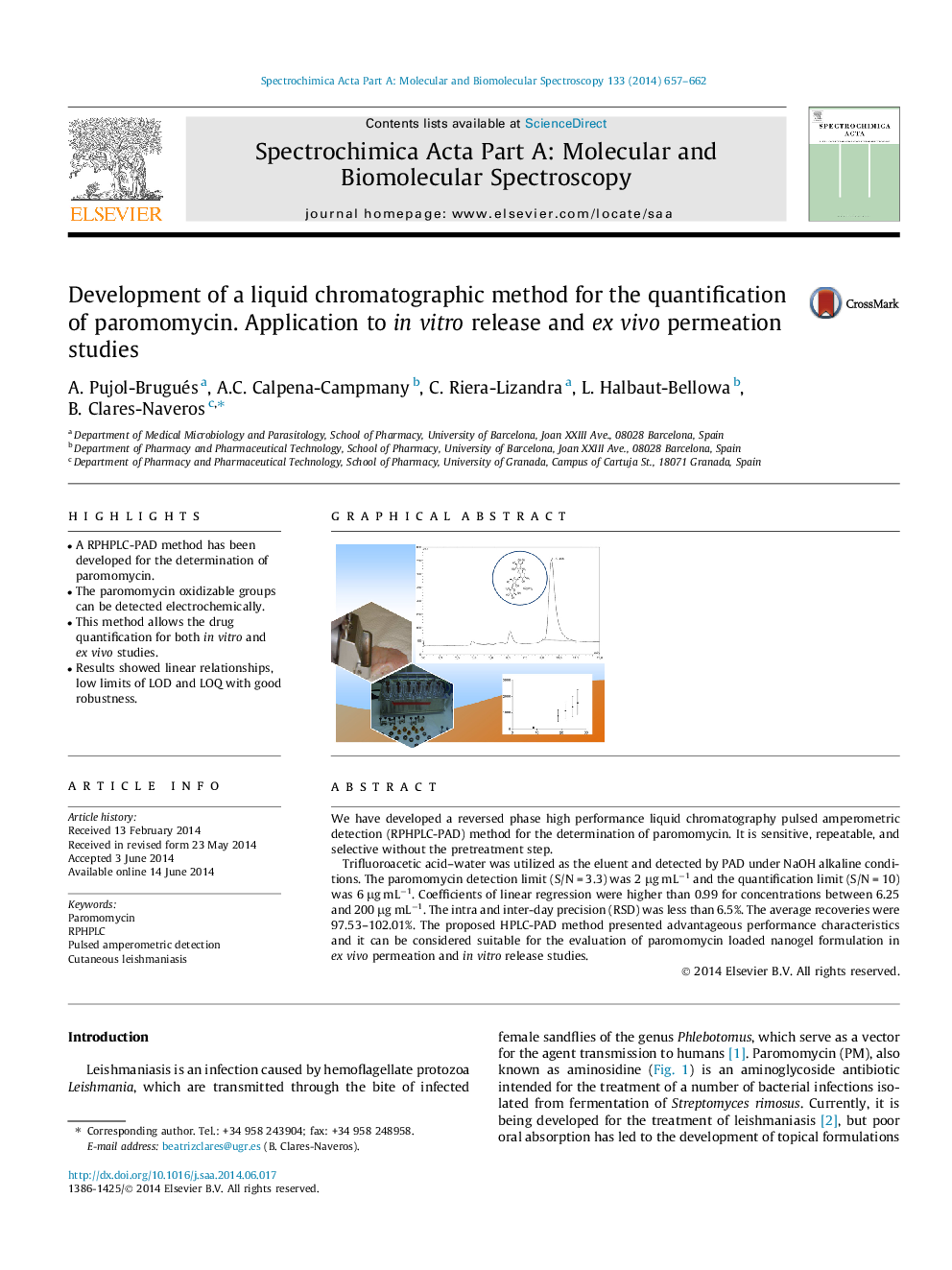
• A RPHPLC-PAD method has been developed for the determination of paromomycin.
• The paromomycin oxidizable groups can be detected electrochemically.
• This method allows the drug quantification for both in vitro and ex vivo studies.
• Results showed linear relationships, low limits of LOD and LOQ with good robustness.
We have developed a reversed phase high performance liquid chromatography pulsed amperometric detection (RPHPLC-PAD) method for the determination of paromomycin. It is sensitive, repeatable, and selective without the pretreatment step.Trifluoroacetic acid–water was utilized as the eluent and detected by PAD under NaOH alkaline conditions. The paromomycin detection limit (S/N = 3.3) was 2 μg mL−1 and the quantification limit (S/N = 10) was 6 μg mL−1. Coefficients of linear regression were higher than 0.99 for concentrations between 6.25 and 200 μg mL−1. The intra and inter-day precision (RSD) was less than 6.5%. The average recoveries were 97.53–102.01%. The proposed HPLC-PAD method presented advantageous performance characteristics and it can be considered suitable for the evaluation of paromomycin loaded nanogel formulation in ex vivo permeation and in vitro release studies.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 133, 10 December 2014, Pages 657–662