| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233497 | 1495235 | 2014 | 6 صفحه PDF | دانلود رایگان |
• New N-butyl bis-benzimidazolyl diamide ligand has been synthesized.
• Copper(II) metallatriangle with ligand sharing are structurally characterized.
• Variable temp magnetic data shows very weak magnetic exchange interaction.
• Oxidation of substituted phenol yields phenoxazine and phenoxazinone.
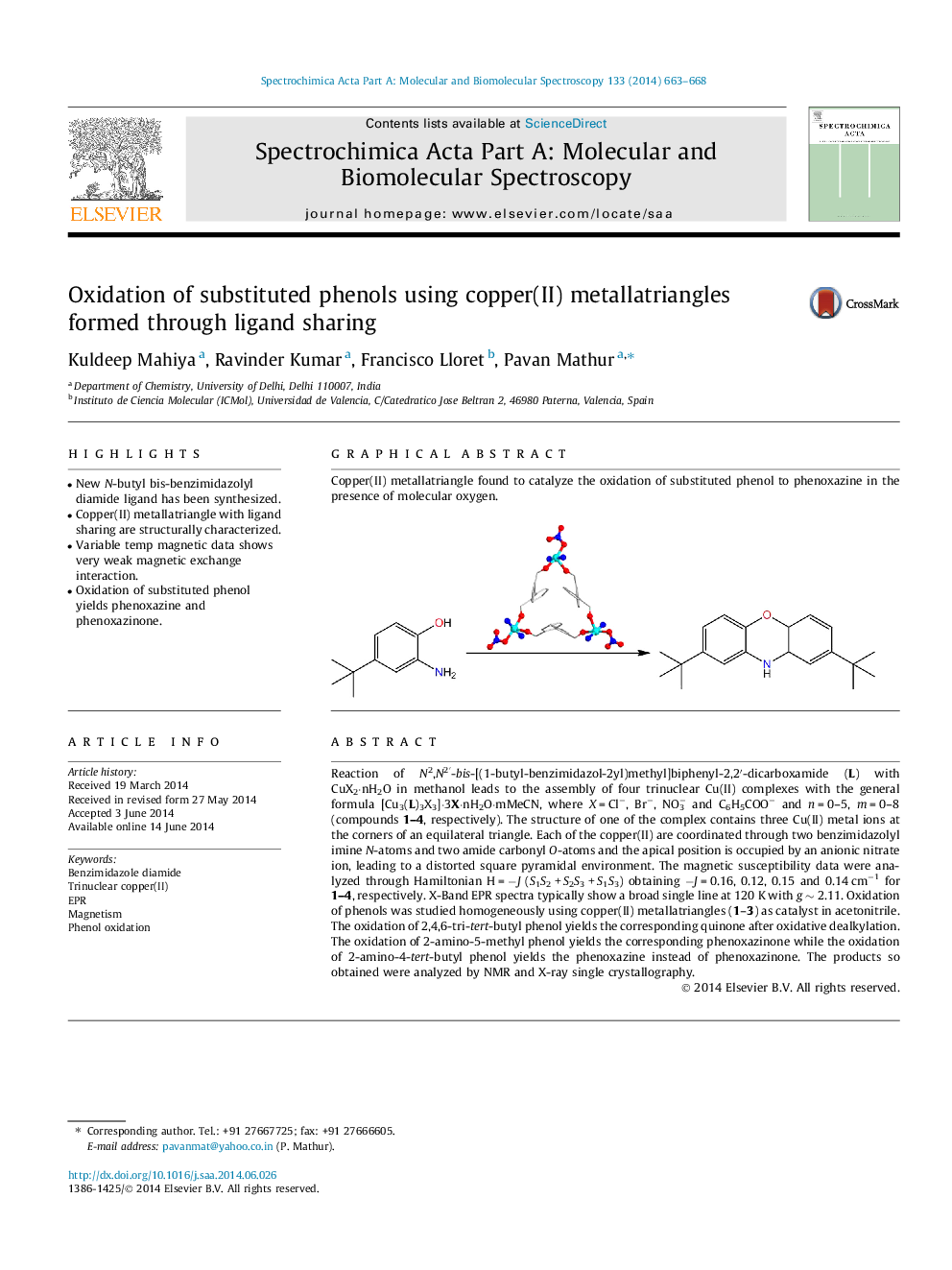
Reaction of N2,N2′-bis-[(1-butyl-benzimidazol-2yl)methyl]biphenyl-2,2′-dicarboxamide (L) with CuX2⋅nH2O in methanol leads to the assembly of four trinuclear Cu(II) complexes with the general formula [Cu3(L)3X3]⋅3X⋅nH2O⋅mMeCN, where X = Cl−, Br−, NO3− and C6H5COO− and n = 0–5, m = 0–8 (compounds 1–4, respectively). The structure of one of the complex contains three Cu(II) metal ions at the corners of an equilateral triangle. Each of the copper(II) are coordinated through two benzimidazolyl imine N-atoms and two amide carbonyl O-atoms and the apical position is occupied by an anionic nitrate ion, leading to a distorted square pyramidal environment. The magnetic susceptibility data were analyzed through Hamiltonian H = −J (S1S2 + S2S3 + S1S3) obtaining −J = 0.16, 0.12, 0.15 and 0.14 cm−1 for 1–4, respectively. X-Band EPR spectra typically show a broad single line at 120 K with g ∼ 2.11. Oxidation of phenols was studied homogeneously using copper(II) metallatriangles (1–3) as catalyst in acetonitrile. The oxidation of 2,4,6-tri-tert-butyl phenol yields the corresponding quinone after oxidative dealkylation. The oxidation of 2-amino-5-methyl phenol yields the corresponding phenoxazinone while the oxidation of 2-amino-4-tert-butyl phenol yields the phenoxazine instead of phenoxazinone. The products so obtained were analyzed by NMR and X-ray single crystallography.
Copper(II) metallatriangle found to catalyze the oxidation of substituted phenol to phenoxazine in the presence of molecular oxygen.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 133, 10 December 2014, Pages 663–668