| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1233500 | 1495235 | 2014 | 14 صفحه PDF | دانلود رایگان |
• The experimental parameters were compared with DFT-B3LYP calculations.
• Solvent effects on the geometry and electronic properties of 3M3HI4C-A were studied.
• NBO analysis, HOMO and LUMO energies confirm that charge transfer occurs in 3M3HI4C-A.
• Molecular ESP map predicts the most attractive site for electrophilic attack.
• The vibrational analysis of title compound was performed in gas phase and in solvents.
FT-IR and FT-Raman spectra of 3-methyl-3h-imidazole-4-carbaldehyde (3M3HI4C) were recorded in the region 4000–400 cm−1 and 3500–50 cm−1, respectively. Optimized geometric parameters, conformational equilibria, normal mode frequencies, and corresponding vibrational assignments of 3M3HI4C were theoretically examined by quantum chemical methods for the first time. All vibrational frequencies were assigned in detail with the help of total energy distribution (TEDs). The experimental wavenumbers were compared with the scaled vibrational frequencies determined by DFT/B3LYP method. The results showed that the B3LYP/6-311++G(d,p) method predicts vibrational frequencies and the structural parameters effectively. The most stable conformer of the title compound was determined. The total electron density and molecular electrostatic potential surfaces of the molecule were constructed by using B3LYP/6-311++G(d,p) method to display electrostatic potential (electron + nuclei) distribution. The electronic properties HOMO and LUMO energies were measured. The lower energy band was assigned to the HOMO → LUMO transition. Natural bond orbital analysis of title molecule has been performed to indicate the presence of intramolecular charge transfer. Energies, relative stabilities, and dipole moments of title molecule were also compared and analyzed in the gas phase and in solvents. Furthermore, solvent effects on the geometry and vibrational frequency of 3M3HI4C were studied theoretically at the DFT/B3LYP level in combination with the conductor polarizable continuum model (C-PCM).
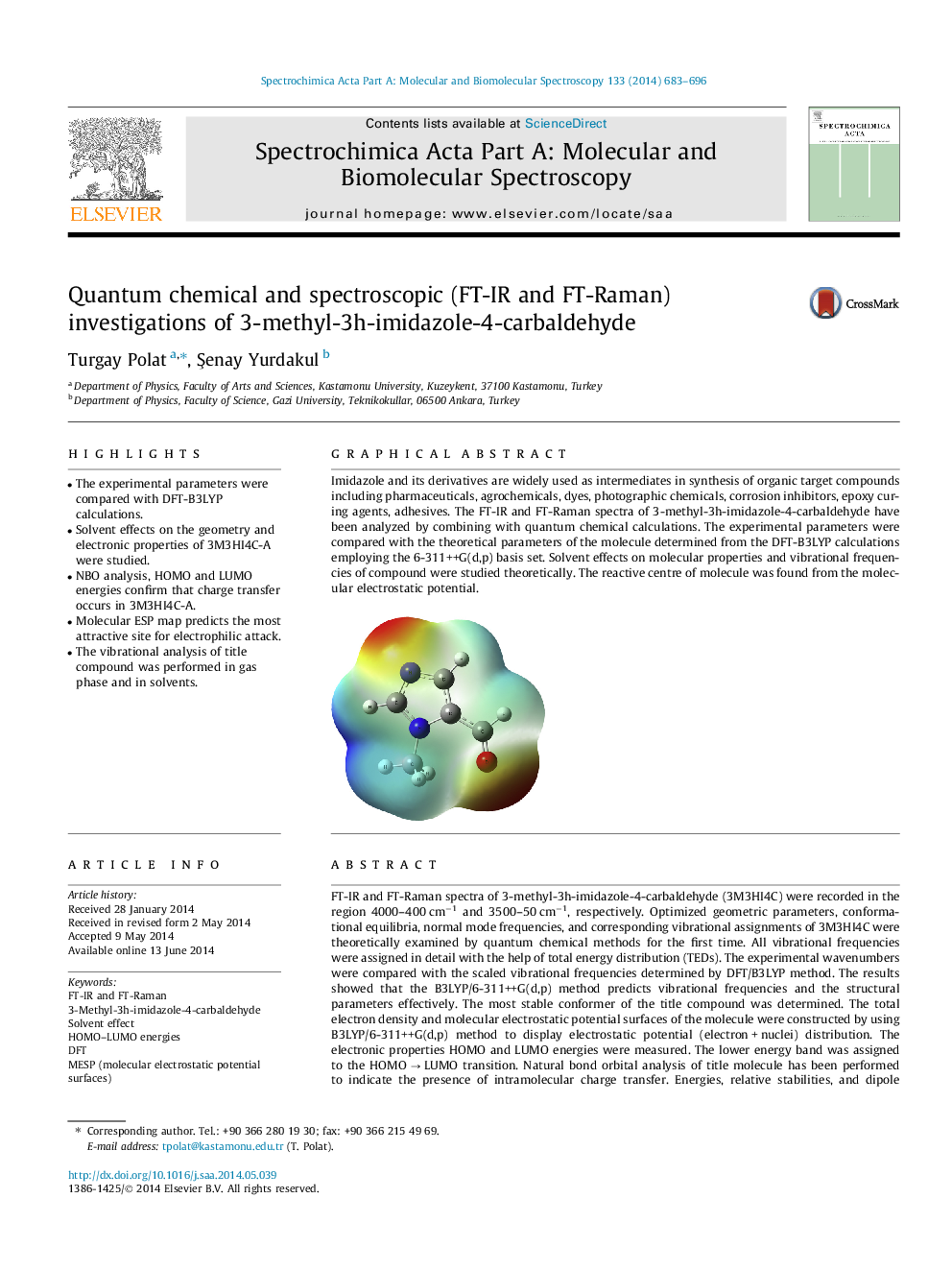
Imidazole and its derivatives are widely used as intermediates in synthesis of organic target compounds including pharmaceuticals, agrochemicals, dyes, photographic chemicals, corrosion inhibitors, epoxy curing agents, adhesives. The FT-IR and FT-Raman spectra of 3-methyl-3h-imidazole-4-carbaldehyde have been analyzed by combining with quantum chemical calculations. The experimental parameters were compared with the theoretical parameters of the molecule determined from the DFT-B3LYP calculations employing the 6-311++G(d,p) basis set. Solvent effects on molecular properties and vibrational frequencies of compound were studied theoretically. The reactive centre of molecule was found from the molecular electrostatic potential.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 133, 10 December 2014, Pages 683–696