| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1234461 | 1495272 | 2012 | 7 صفحه PDF | دانلود رایگان |
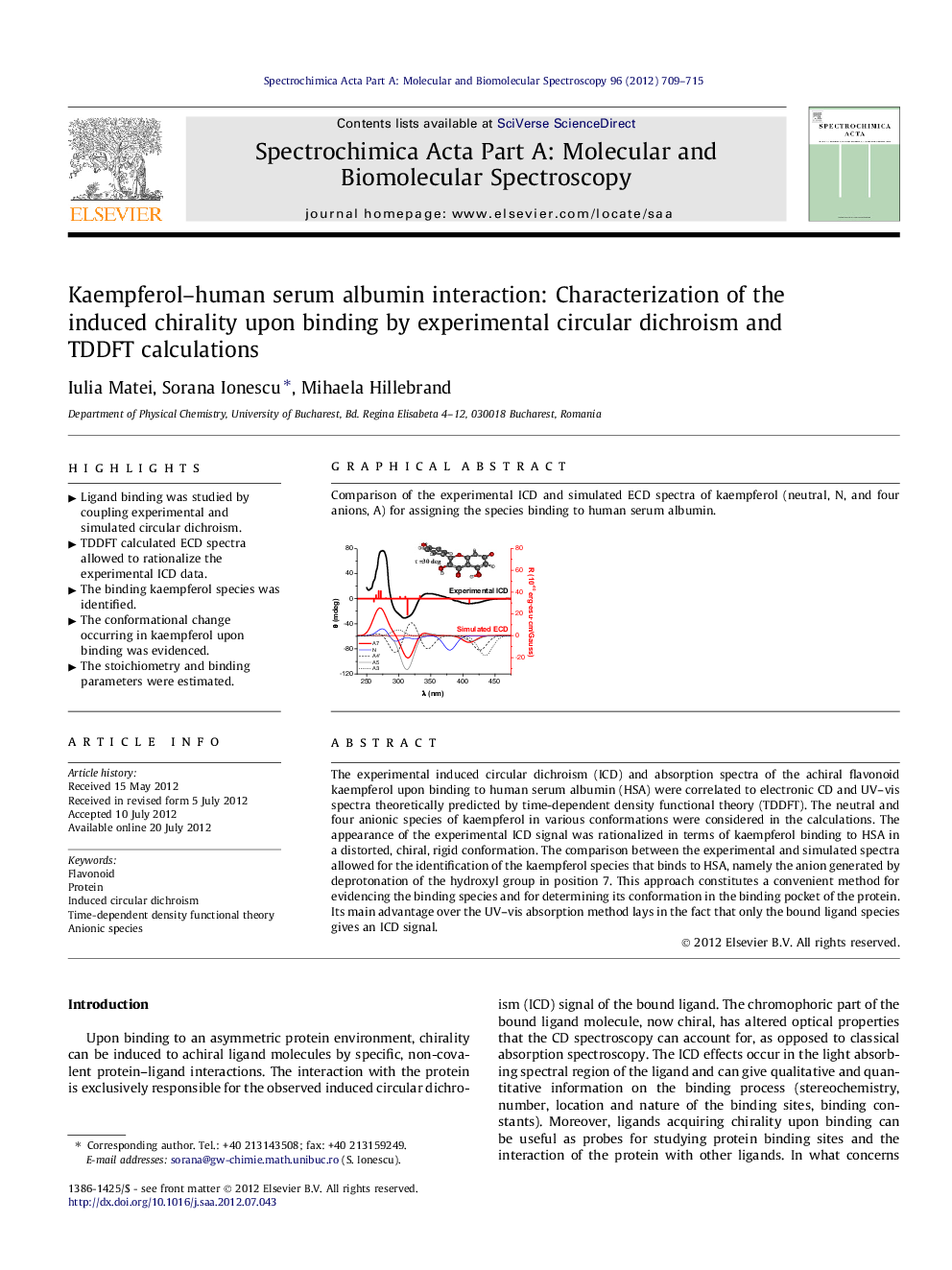
The experimental induced circular dichroism (ICD) and absorption spectra of the achiral flavonoid kaempferol upon binding to human serum albumin (HSA) were correlated to electronic CD and UV–vis spectra theoretically predicted by time-dependent density functional theory (TDDFT). The neutral and four anionic species of kaempferol in various conformations were considered in the calculations. The appearance of the experimental ICD signal was rationalized in terms of kaempferol binding to HSA in a distorted, chiral, rigid conformation. The comparison between the experimental and simulated spectra allowed for the identification of the kaempferol species that binds to HSA, namely the anion generated by deprotonation of the hydroxyl group in position 7. This approach constitutes a convenient method for evidencing the binding species and for determining its conformation in the binding pocket of the protein. Its main advantage over the UV–vis absorption method lays in the fact that only the bound ligand species gives an ICD signal.
Comparison of the experimental ICD and simulated ECD spectra of kaempferol (neutral, N, and four anions, A) for assigning the species binding to human serum albumin.Figure optionsDownload as PowerPoint slideHighlights
► Ligand binding was studied by coupling experimental and simulated circular dichroism.
► TDDFT calculated ECD spectra allowed to rationalize the experimental ICD data.
► The binding kaempferol species was identified.
► The conformational change occurring in kaempferol upon binding was evidenced.
► The stoichiometry and binding parameters were estimated.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 96, October 2012, Pages 709–715