| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1284287 | 1497986 | 2014 | 8 صفحه PDF | دانلود رایگان |
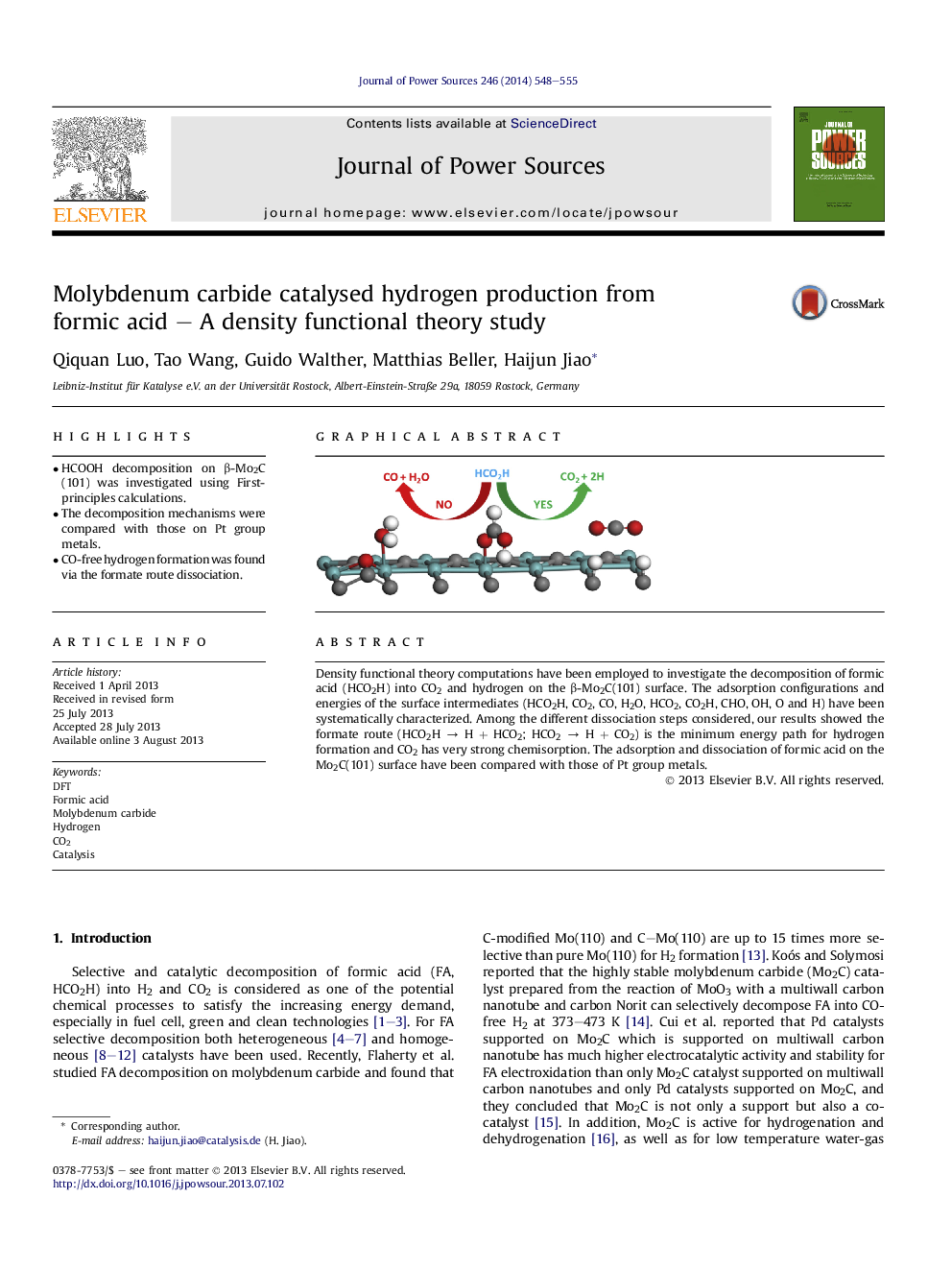
• HCOOH decomposition on β-Mo2C(101) was investigated using First-principles calculations.
• The decomposition mechanisms were compared with those on Pt group metals.
• CO-free hydrogen formation was found via the formate route dissociation.
Density functional theory computations have been employed to investigate the decomposition of formic acid (HCO2H) into CO2 and hydrogen on the β-Mo2C(101) surface. The adsorption configurations and energies of the surface intermediates (HCO2H, CO2, CO, H2O, HCO2, CO2H, CHO, OH, O and H) have been systematically characterized. Among the different dissociation steps considered, our results showed the formate route (HCO2H → H + HCO2; HCO2 → H + CO2) is the minimum energy path for hydrogen formation and CO2 has very strong chemisorption. The adsorption and dissociation of formic acid on the Mo2C(101) surface have been compared with those of Pt group metals.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 246, 15 January 2014, Pages 548–555