| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1285362 | 1497918 | 2016 | 8 صفحه PDF | دانلود رایگان |
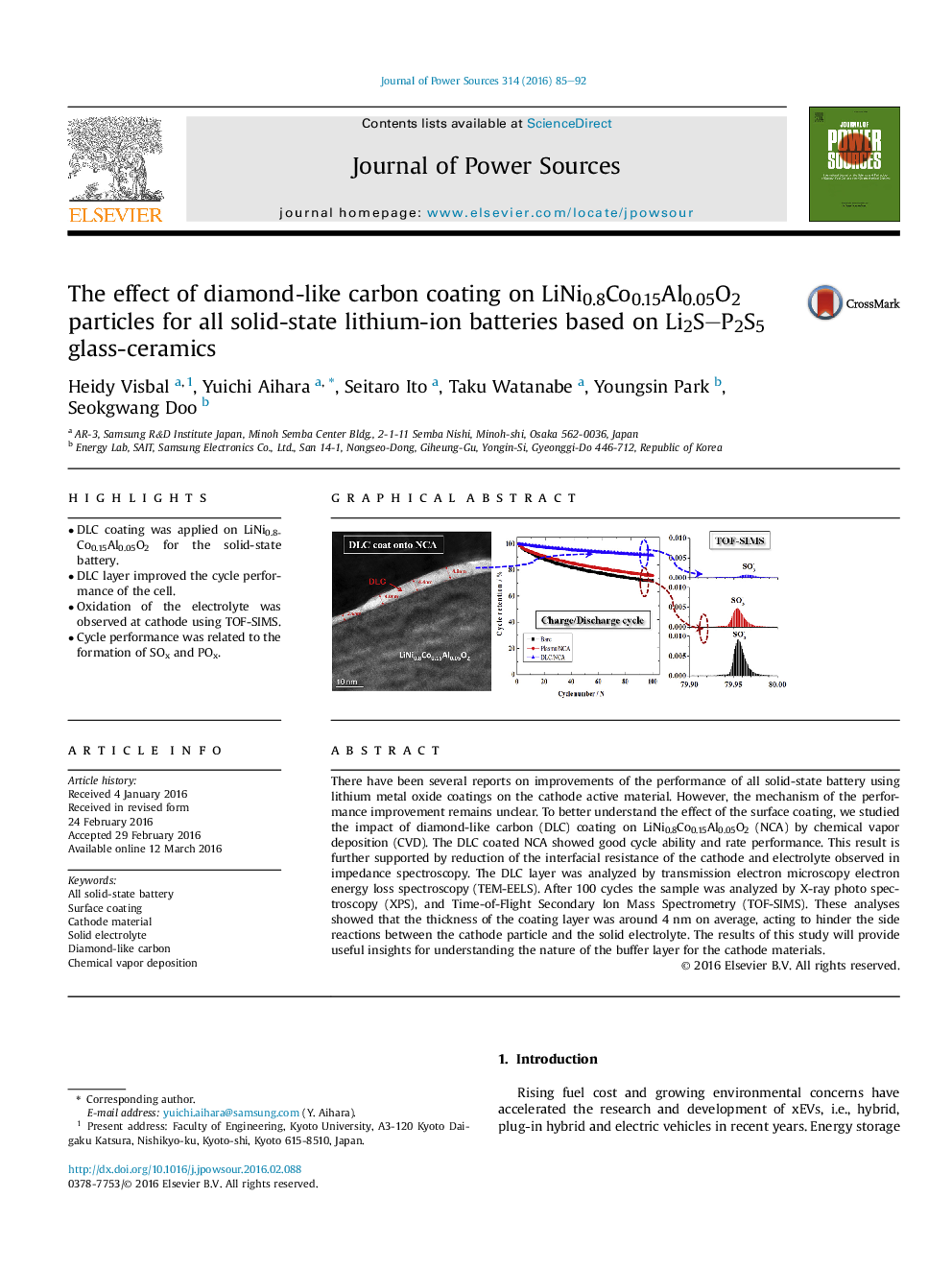
• DLC coating was applied on LiNi0.8Co0.15Al0.05O2 for the solid-state battery.
• DLC layer improved the cycle performance of the cell.
• Oxidation of the electrolyte was observed at cathode using TOF-SIMS.
• Cycle performance was related to the formation of SOx and POx.
There have been several reports on improvements of the performance of all solid-state battery using lithium metal oxide coatings on the cathode active material. However, the mechanism of the performance improvement remains unclear. To better understand the effect of the surface coating, we studied the impact of diamond-like carbon (DLC) coating on LiNi0.8Co0.15Al0.05O2 (NCA) by chemical vapor deposition (CVD). The DLC coated NCA showed good cycle ability and rate performance. This result is further supported by reduction of the interfacial resistance of the cathode and electrolyte observed in impedance spectroscopy. The DLC layer was analyzed by transmission electron microscopy electron energy loss spectroscopy (TEM-EELS). After 100 cycles the sample was analyzed by X-ray photo spectroscopy (XPS), and Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS). These analyses showed that the thickness of the coating layer was around 4 nm on average, acting to hinder the side reactions between the cathode particle and the solid electrolyte. The results of this study will provide useful insights for understanding the nature of the buffer layer for the cathode materials.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 314, 15 May 2016, Pages 85–92