| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1286248 | 1497919 | 2016 | 8 صفحه PDF | دانلود رایگان |
• I− substituted LiFe0.4Mn0.6(PO4) as cathode material fabricated for the first time.
• LiFe0.4Mn0.6(PO4)1−xIx showed enhanced rate performance at various C-rate.
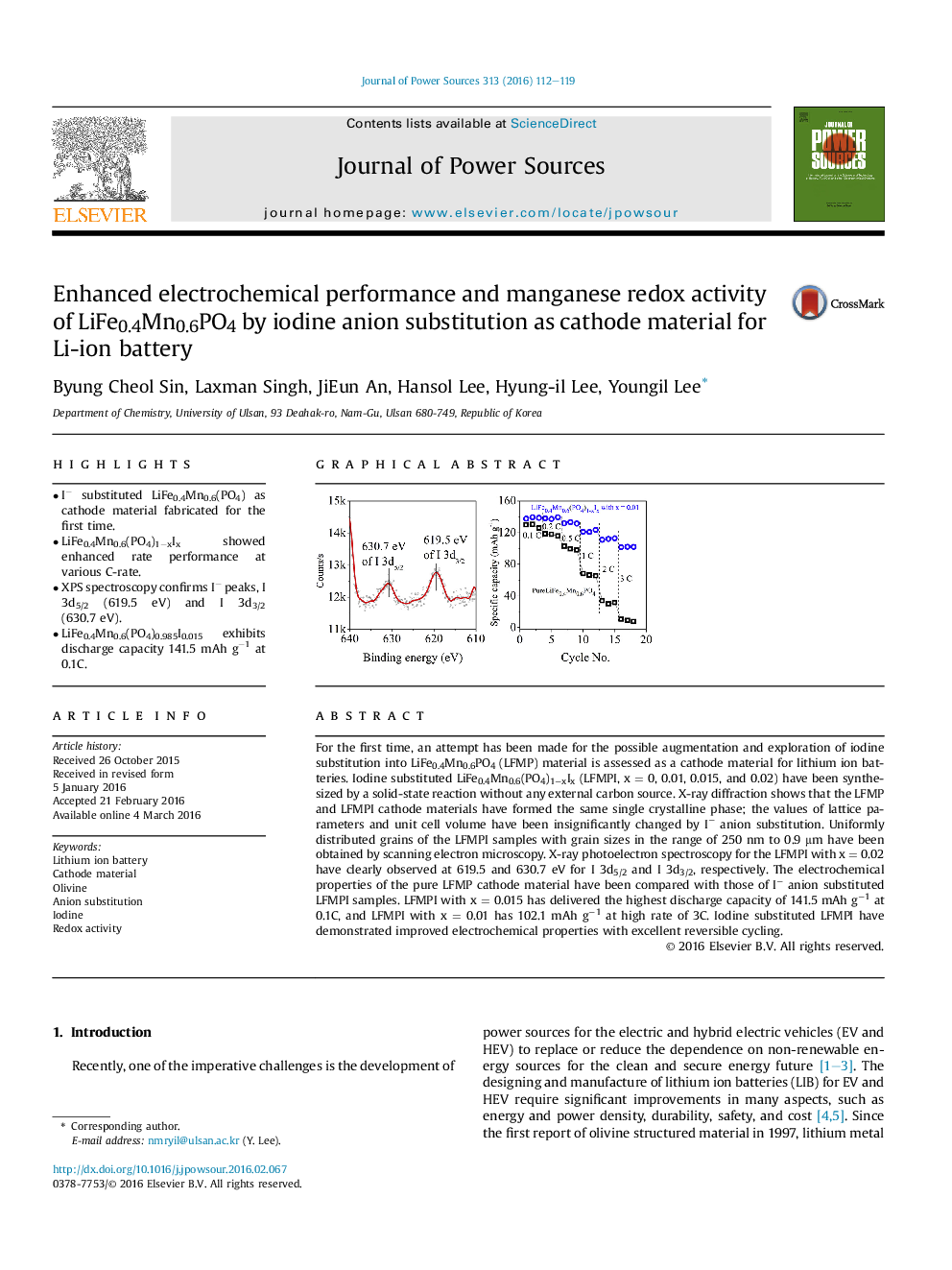
• XPS spectroscopy confirms I− peaks, I 3d5/2 (619.5 eV) and I 3d3/2 (630.7 eV).
• LiFe0.4Mn0.6(PO4)0.985I0.015 exhibits discharge capacity 141.5 mAh g−1 at 0.1C.
For the first time, an attempt has been made for the possible augmentation and exploration of iodine substitution into LiFe0.4Mn0.6PO4 (LFMP) material is assessed as a cathode material for lithium ion batteries. Iodine substituted LiFe0.4Mn0.6(PO4)1−xIx (LFMPI, x = 0, 0.01, 0.015, and 0.02) have been synthesized by a solid-state reaction without any external carbon source. X-ray diffraction shows that the LFMP and LFMPI cathode materials have formed the same single crystalline phase; the values of lattice parameters and unit cell volume have been insignificantly changed by I− anion substitution. Uniformly distributed grains of the LFMPI samples with grain sizes in the range of 250 nm to 0.9 μm have been obtained by scanning electron microscopy. X-ray photoelectron spectroscopy for the LFMPI with x = 0.02 have clearly observed at 619.5 and 630.7 eV for I 3d5/2 and I 3d3/2, respectively. The electrochemical properties of the pure LFMP cathode material have been compared with those of I− anion substituted LFMPI samples. LFMPI with x = 0.015 has delivered the highest discharge capacity of 141.5 mAh g−1 at 0.1C, and LFMPI with x = 0.01 has 102.1 mAh g−1 at high rate of 3C. Iodine substituted LFMPI have demonstrated improved electrochemical properties with excellent reversible cycling.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 313, 1 May 2016, Pages 112–119